Pathios Gains $25M for Immunotherapy Trials in Series B Funding
Pathios Therapeutics Limited, a biotechnological firm dedicated to creating innovative cancer treatments, disclosed that it has secured $25 million during the initial phase of its Series B fundraising round. The round features a key new investment from Bristol Myers Squibb and continued backing from prior investors such as Canaan and Brandon Capital.
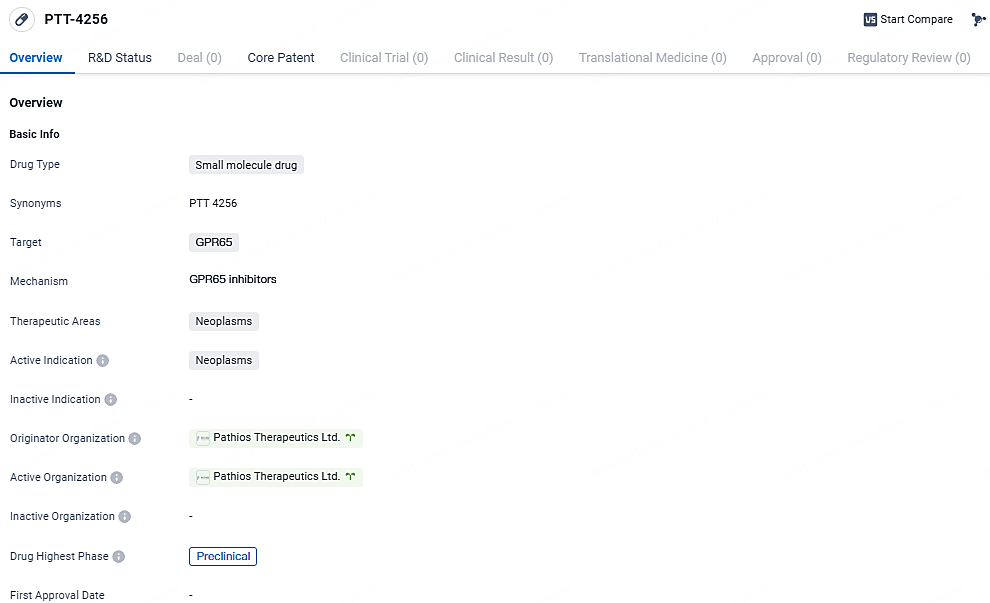
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The funding obtained will be allocated to further develop Pathios’ innovative strategy in cancer immunotherapy, which is centered on blocking GPR65, an emerging target linked to a spectrum of immunological diseases. Pathios plans to use the funds to push forward its primary candidate, PTT-4256, into clinical trials for advanced solid tumors by the end of 2024. This compound is a powerful, selective small molecule and is administered orally.
Furthermore, the financial influx will enable Pathios to enhance its workforce, particularly at the executive level, as it transitions into a clinical-stage entity. Tom McCarthy, Ph.D., co-founder and executive chair of Pathios, stated, “With the backing from top-tier life science investors such as Canaan, Brandon Capital, and notable oncology leader Bristol Myers Squibb, we gain both capital and valuable insights. Our aim is to validate clinically that inhibiting GPR65 could herald a transformative era in immunotherapy."
He also expressed his appreciation for the investors’ faith in their scientific pursuit and its broad applicative potential in oncology and other immune-based disorders. McCarthy is optimistic about utilizing this capital to progress their clinical activities, particularly introducing PTT-4256 into clinical settings later this year.
At the American Association for Cancer Research Annual Meeting 2023, preliminary data showcased PTT-4256’s promising characteristics as a drug and its efficacy as a monotherapy in combating tumors. Notably effective at modifying the acidic environment that drives immunosuppression in macrophages, PTT-4256 also prompted increases in pro-inflammatory genes linked to anti-tumor responses. This was displayed alongside elevated T cell and natural killer cell activities within the tumor microenvironment (TME), alongside suppressing pro-tumor cytokines. Such comprehensive effects underscored PTT-4256's standalone therapeutic potential in syngeneic mouse models of cancer via oral administration.
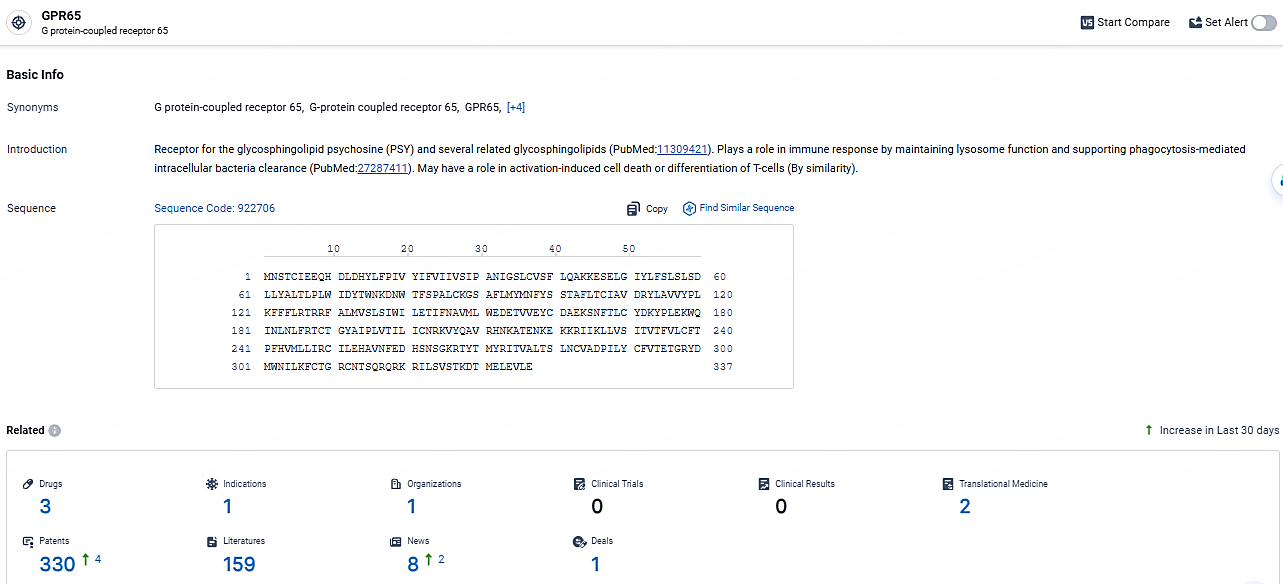
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 22, 2024, there are 3 investigational drugs for the GPR65 target, including 1 indications, 1 R&D institutions involved, and as many as 330 patents.
PTT-4256 targets GPR65 in neoplasms. It is currently in the preclinical phase of development, and more research and clinical trials will be required to evaluate its potential as a treatment for this disease.