miRNA and Disease Regulation
miRNA is an endogenous tiny non-coding RNA, generally 21-23 nucleotides long, with the function of regulating gene expression at the translational level. The occurrence and "growth" of miRNAs require several steps: First, the miRNA gene is transcribed by RNA polymerase to produce a longer precursor miRNA (pri-miRNA), which then forms a hairpin-shaped pre-miRNA. Finally, it is cut in the cytoplasm to form a mature miRNA duplex. Thus, miRNA transforms from a >1000nt "cutie" into a roughly 22nt "elf". Mature miRNAs are loaded onto AGO proteins, combining to form an active RNA-induced silencing complex (RISC), playing their various regulatory functions.
Mediating gene silencing in the cytoplasm is the "skill" of miRNA. Given the low complementarity of miRNAs, miRNAs often imperfectly pair with target genes, therefore, they can regulate the expression of multiple target genes at the same time. It is estimated that miRNAs are involved in various key biological and cellular processes such as apoptosis, differentiation, development, proliferation, metabolism, and signal transduction pathways, regulating up to 30% of protein-coding genes in the human genome. Additionally, miRNA dysregulation is often associated with various human diseases such as cancer, diabetes, and cardiovascular diseases. With in-depth research on miRNAs, it has been found that miRNAs can target multiple mRNAs that change under disease conditions using miRNA mimics, miRNA inhibitors, or other forms for various disease treatments.
MiRNA Competitive Landscape
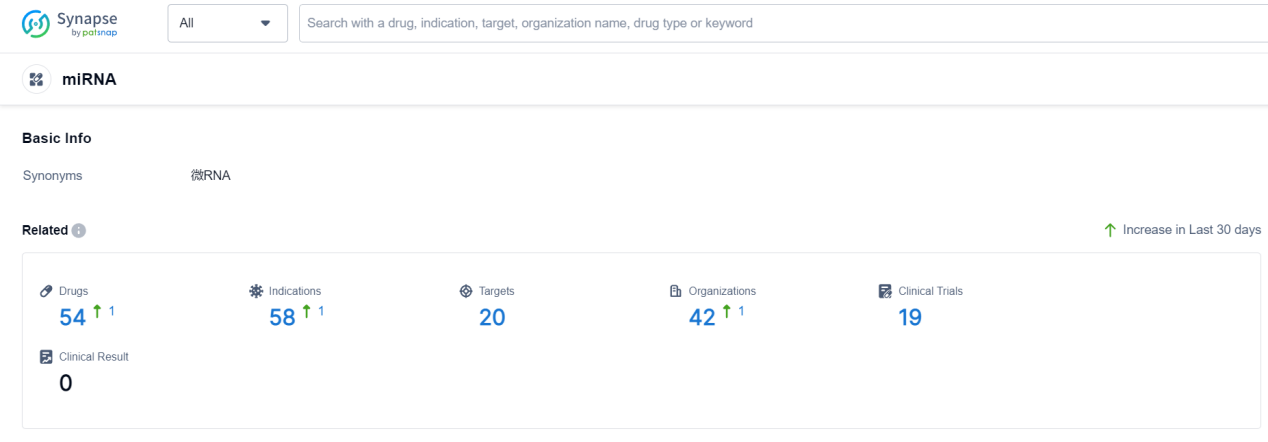
According to Patsnap Synapse, as of 19 Sep 2023, there are a total of 54 miRNA drugs worldwide, from 42 organizations, covering 20 targets, 58 indications, and conducting 19 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts,you can browse the latest research progress on drugs,indications,organizations,clinical trials,clinical results,and drug patents related to this drug type.
Phase II Clinical Trial of miRNA Medicinal: Cobomarsen
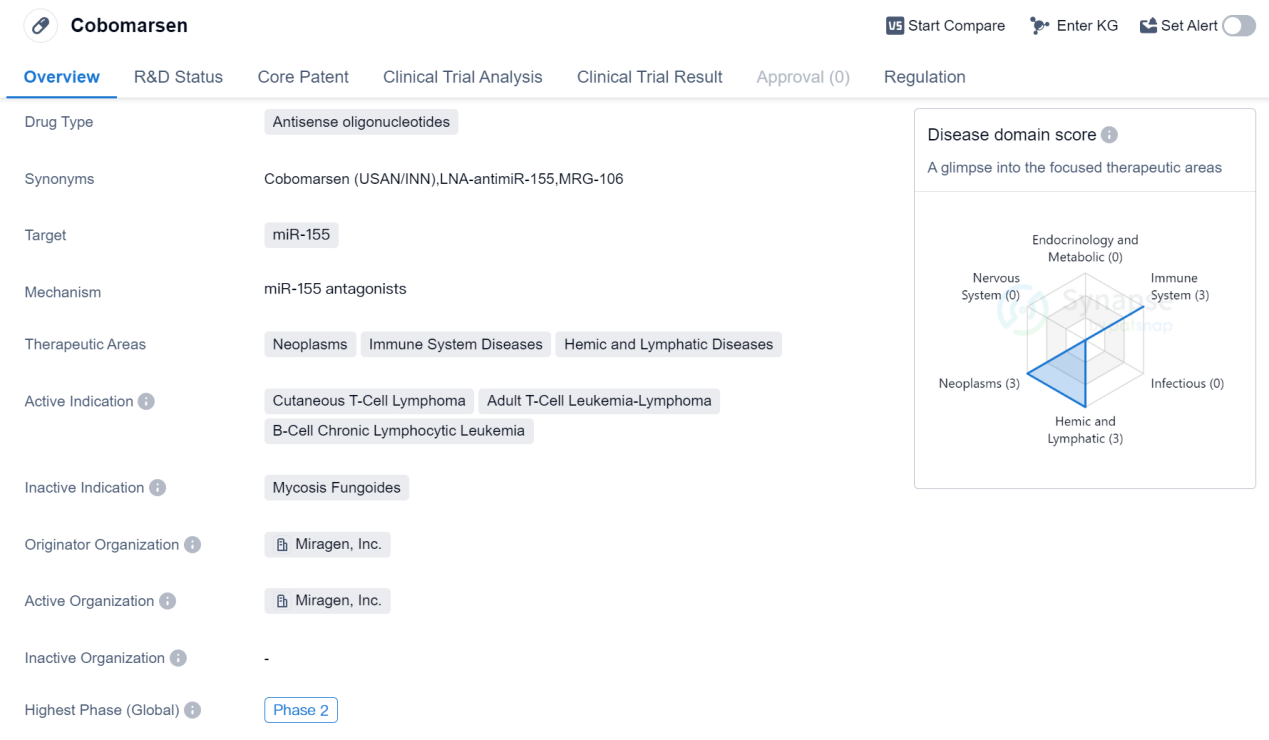
Cobomarsen is an antisense oligonucleotide drug that is being developed by Miragen, Inc. It falls under the therapeutic area of neoplasms, immune system diseases, and hemic and lymphatic diseases. The drug specifically targets miR-155, a microRNA that has been implicated in various diseases, including cutaneous T-cell lymphoma, adult T-cell leukemia-lymphoma, and B-cell chronic lymphocytic leukemia.
Currently, Cobomarsen is in its highest phase of development, which is Phase 2. This means that the drug has already undergone initial testing for safety and efficacy and is now being evaluated in a larger group of patients to further assess its effectiveness and potential side effects. Phase 2 trials are crucial in determining the drug's overall benefit-risk profile and its potential for further development.
One notable aspect of Cobomarsen is its regulatory status as an orphan drug. Orphan drugs are medications that are developed to treat rare diseases or conditions that affect a small number of patients. The designation of orphan drug status provides certain incentives and benefits to the drug developer, such as market exclusivity and financial incentives, to encourage the development of treatments for rare diseases.
👇Please click on the image below to directly access the latest data(R&D Status|Core Patent|Clinical Trial|Approval status in Global countries)of this drug.
Overall, Cobomarsen shows promise as a potential treatment for cutaneous T-cell lymphoma, adult T-cell leukemia-lymphoma, and B-cell chronic lymphocytic leukemia. Further research and clinical trials are needed to fully evaluate the safety and efficacy of Cobomarsen before it can be approved for widespread use in patients.