Mission Therapeutics announces FDA approval for Phase II trial of their lead drug, MTX652, aimed at treating Acute Kidney Injury
Mission Therapeutics, an emerging leader in the biopharmaceutical sector, is advancing a pioneering drug development program aimed at regulating mitophagy. The company has shared news that the US Food and Drug Administration (FDA) has given the green light to its IND application for MTX652. This milestone marks a pivotal step forward, granting Mission Therapeutics the authorization to initiate its anticipated Phase II clinical trial for MTX652 within the United States.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Aiming to enroll around 160 participants who have a heightened susceptibility to Acute Kidney Injury post-cardiac surgery, the study plans to span across various locations throughout North America and Europe. The trial's format is a double-blinded, placebo-managed design and anticipates its inauguration for early in the forthcoming year, with a focus on demonstrating that MTX652 brings protection against AKI to the patient population at elevated risk. The evaluation will include monitoring established renal function and injury indicators over a period.
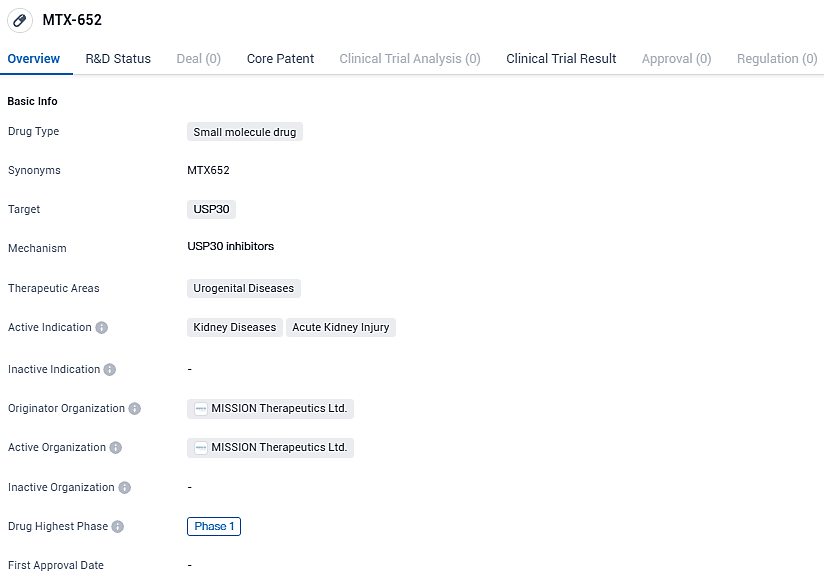
The CEO of Mission Therapeutics, Dr. Anker Lundemose, commented: "Securing the FDA's endorsement for our Phase II investigation of our leading compound, MTX652, signifies a pivotal advancement for Mission. Our progress now encompasses two USP30 inhibitors in clinical development—MTX652 for acute kidney injury and MTX325 for Parkinson's Disease—affirming the efficacy of our distinctive strategy and our extensive portfolio of compounds."
Dr. Suhail Nurbhai, the CMO of Mission Therapeutics, remarked: "Given the absence of sanctioned medicinal therapies and the potential for grave immediate and long-term impacts, such as ongoing renal function deterioration or the possible need for renal replacement therapy, we are confident in MTX562's potential to mitigate these negative effects and address the critical and unmet medical necessity. Receiving this authorization is a cause for celebration, and we eagerly anticipate the trial's commencement in 2024."
This FDA approval came on the heels of a Phase I First Time in Human study's completion earlier in 2023, where MTX652 was administered to over 80 healthy individuals. The trial outcomes showed that MTX652 was secure and well-received, with successful administration of up to 200mg as a singled-out dosage and repeated 100mg daily doses over a two-week timeframe, coupled with an exemplary pharmacokinetic profile.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
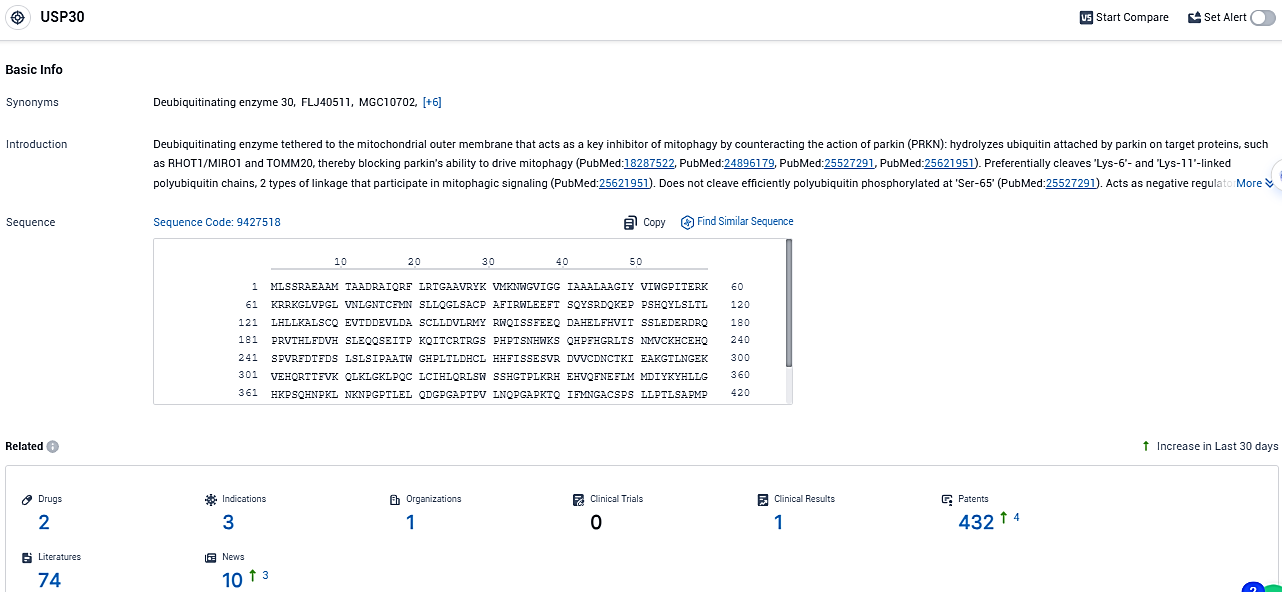
According to the data provided by the Synapse Database, As of December 23, 2023, there are 2 investigational drugs for the USP30 target, including 3 indications, 1 R&D institutions involved, and as many as 432 patents.
MTX652 is a potent and selective compound designed to improve mitochondrial quality and function by enhancing mitophagy through inhibition of USP30, a deubiquitylating enzyme localised to mitochondria which is a negative regulator of mitophagy. MTX652 has the potential to reduce the impact of impaired mitochondria associated with Ischaemic Reperfusion Injury (IRI) in the heart and kidneys in patients who have undergone heart surgery.