Sumitomo Pharma Renews Collaborative Development Agreement with Otsuka Pharmaceutical for Multiple Psychiatric Disorder Treatments
On March 15th, Sumitomo Dainippon Pharma Co., Ltd., and its U.S. subsidiary, Sumitomo Pharma America, Inc. (SMPA), announced that they have amended the global collaboration and commercialization agreement originally signed with Sumitomo Dainippon Pharma, SMPA, and Otsuka Pharmaceutical Co., Ltd., on September 30, 2021, concerning the joint development of four investigational compounds in the fields of psychiatry and neurology, including ulotaront, SEP-380135, SEP-4199, and SEP-378614 (hereinafter referred to as "the Amendment Agreement").
It is reported that SEP-4199 and SEP-378614 from the original collaboration agreement will no longer be included in the scope of this Amendment Agreement. SMPA has granted Otsuka Pharmaceutical exclusive global rights to develop, produce, and market ulotaront and SEP-380135 for all indications. SMPA is entitled to receive milestone payments of up to 30 million U.S. dollars (approximately 45 billion Japanese yen) related to the development progress of ulotaront and SEP-380135 from Otsuka Pharmaceutical, as well as royalties based on Otsuka Pharmaceutical's sales revenue.
Sumitomo Pharma and SMPA have been collaborating with Otsuka Pharmaceutical in the development of novel candidate compounds including ulotaront in the priority areas of psychiatry and neurology. However, given the current situation, the likelihood of these compounds generating revenue during the Mid-Term Business Plan period for fiscal 2027 (FY2023-FY2027) poses significant challenges. In light of this, the Sumitomo Pharma Group has reassessed its priority development products and has decided to focus its efforts on the development of projects in the fields of oncology and regenerative medicine/cell therapy, which are expected to come to market during the Mid-Term Business Plan period for fiscal 2027. Consequently, Sumitomo Pharma Group has transferred the development responsibilities for the aforementioned ulotaront and SEP-380135 to Otsuka Pharmaceutical. At the same time, Sumitomo Pharma Group is evaluating further development strategies for SEP-4199 and SEP-378614.
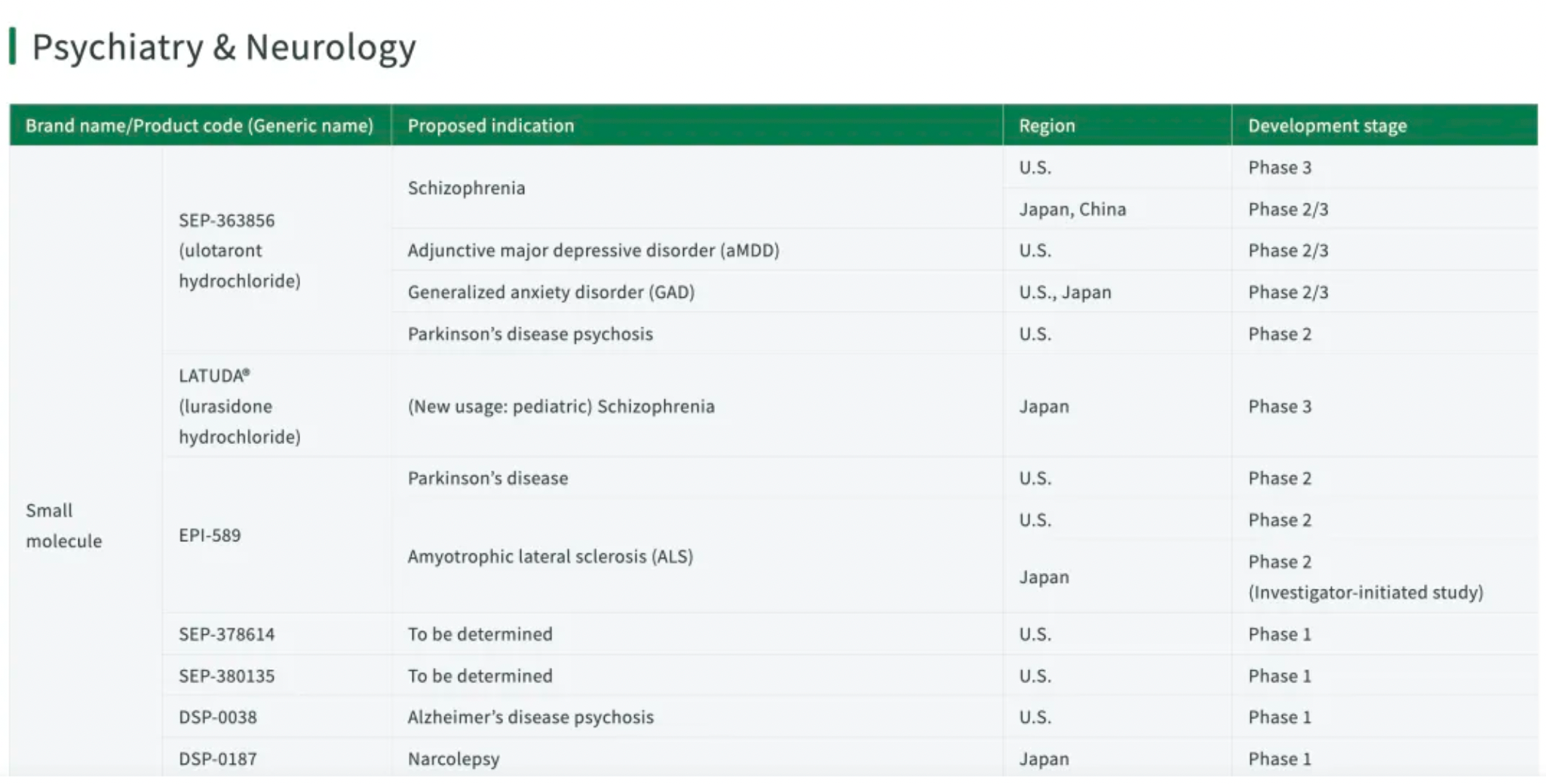
Ulotaront is an agonist associated with Trace Amine-Associated Receptor 1 (TAAR1) and exhibits agonist activity at the 5-HT1A receptor. Currently, ulotaront is in the development phase for the treatment of schizophrenia, as an adjunctive treatment for major depressive disorder (MDD), and for generalized anxiety disorder (GAD).
In May 2019, the U.S. Food and Drug Administration (FDA) granted Breakthrough Therapy designation to Ulotaront for the treatment of schizophrenia, making it the first and only TAAR1 agonist to enter Phase III clinical trials for schizophrenia. Additionally, Ulotaront is the first TAAR1 agonist to progress to Phase II/III clinical trials as an adjunctive treatment for Generalized Anxiety Disorder (GAD) and Major Depressive Disorder (MDD).
However, on July 31, 2023, Sumitomo Pharma announced on its official website that its antipsychotic clinical candidate Ulotaront (SEP-363856) failed to meet the primary endpoints in two recent Phase III clinical studies (DIAMOND 1 and DIAMOND 2).
In April 2020, the team at Sunovion, the U.S. subsidiary of Sumitomo Pharmaceuticals, published the results of a Phase 2 clinical study on the oral small molecule SEP-363856 for the treatment of schizophrenia in The New England Journal of Medicine online.
The study conducted a randomized controlled trial to evaluate the efficacy and safety of SEP-363856 in adult patients with an acute exacerbation of schizophrenia. Patients were randomly assigned in a 1:1 ratio to receive SEP-363856 treatment (either 50 mg or 75 mg) or placebo once daily for a duration of 4 weeks. The primary endpoint was the change in the total score on the Positive and Negative Syndrome Scale (PANSS; range 30 to 210, with higher scores indicating more severe psychotic symptoms) from baseline to week 4.
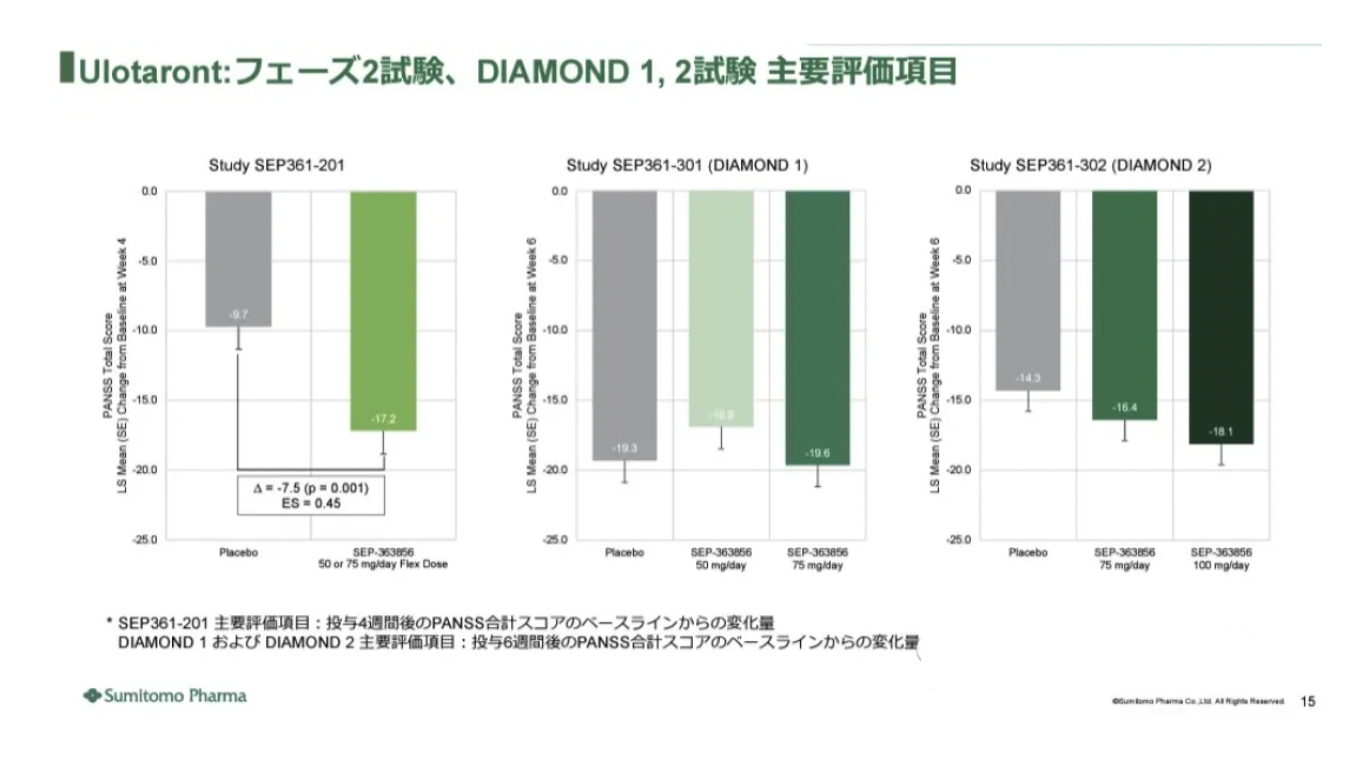
A total of 120 patients were assigned to the SEP-363856 group, and 125 patients were allocated to the placebo group. At baseline, the mean total score on the Positive and Negative Syndrome Scale (PANSS) was 101.4 for the SEP-363856 group and 99.7 for the placebo group. The mean change at week 4 was -17.2 for the SEP-363856 group and -9.7 for the placebo group. The reductions in the Clinical Global Impressions - Severity (CGI-S) and the Brief Negative Symptom Scale (BNSS) scores at week 4 were consistent with the primary outcome in terms of direction of improvement.
The adverse reactions of SEP-363856 include somnolence and gastrointestinal symptoms; a case of sudden cardiac death occurred in the SEP-363856 group. The incidence of extrapyramidal symptoms in the trial group, as well as changes in blood lipid levels, glycated hemoglobin, and prolactin levels, was similar.
In 2021, the Sunovion team published an article in the journal Schizophrenia, reporting on the safety and efficacy of SEP-363856 in the treatment of schizophrenia (results from a 6-month open-label extension study).

According to the study report, this 26-week open-label extension study was designed to evaluate the safety and efficacy of Ulotaront (25/50/75 mg/day) in patients who completed the initial 4-week study. The results showed that after 26 weeks of extended treatment, the mean change in the observed PANSS total score compared to the open-label baseline was -22.6. The long-term treatment with the TAAR1 agonist Ulotaront had a relatively high completion rate, characterized by the absence of extrapyramidal related adverse reactions, a lower incidence of weight and metabolic adverse reactions, and no effect on prolactin levels.
It is reported that the Phase III clinical trial results jointly announced by Sumitomo Pharmaceuticals and Otsuka are multicenter, randomized, double-blind, parallel-group, fixed-dose studies. The DIAMOND 1 study evaluated the efficacy, safety, and tolerability of ulotaront (50 mg/day and 75 mg/day) compared to placebo; while DIAMOND 2 assessed the efficacy, safety, and tolerability of ulotaront (75 mg/day and 100 mg/day) compared to placebo.
In the DIAMOND 1 study, over time, the Positive and Negative Syndrome Scale (PANSS) total scores for all three treatment groups decreased. However, at the primary endpoint of change from baseline in PANSS total score at week 6, the ulotaront treatment groups did not show superiority to the placebo group (patients treated with ulotaront at doses of 50 mg/day and 75 mg/day had changes of -16.9 and -19.6, respectively, compared to -19.3 in the placebo group).
In the DIAMOND 2 study, the 75 mg/day and 100 mg/day ulotaront treatment groups did not show statistically significant improvement over placebo at the primary endpoint. At week 6, the least squares (LS) mean changes for patients treated with 75 mg/day and 100 mg/day ulotaront were -16.4 and -18.1, respectively, compared to -14.3 for the placebo group.
In both DIAMOND 1 and DIAMOND 2 studies, a substantial placebo effect was observed which may have masked the therapeutic effect of the molecule.
SEP-380135 is an orally-administered small molecule drug targeting the central nervous system, jointly developed by SMPA and PsychoGenics, and is currently in Phase I clinical trials in the United States. The discovery of this drug was made possible by the in vivo phenotypic SmartCube® platform and its associated artificial intelligence algorithms.
SmartCube® is a proprietary state-of-the-art automated testing platform by PsychoGenics that uses custom hardware to guide mice through a series of challenges, extracting profound behavioral and physiological insights through integrated computer vision and artificial intelligence (AI) technology.
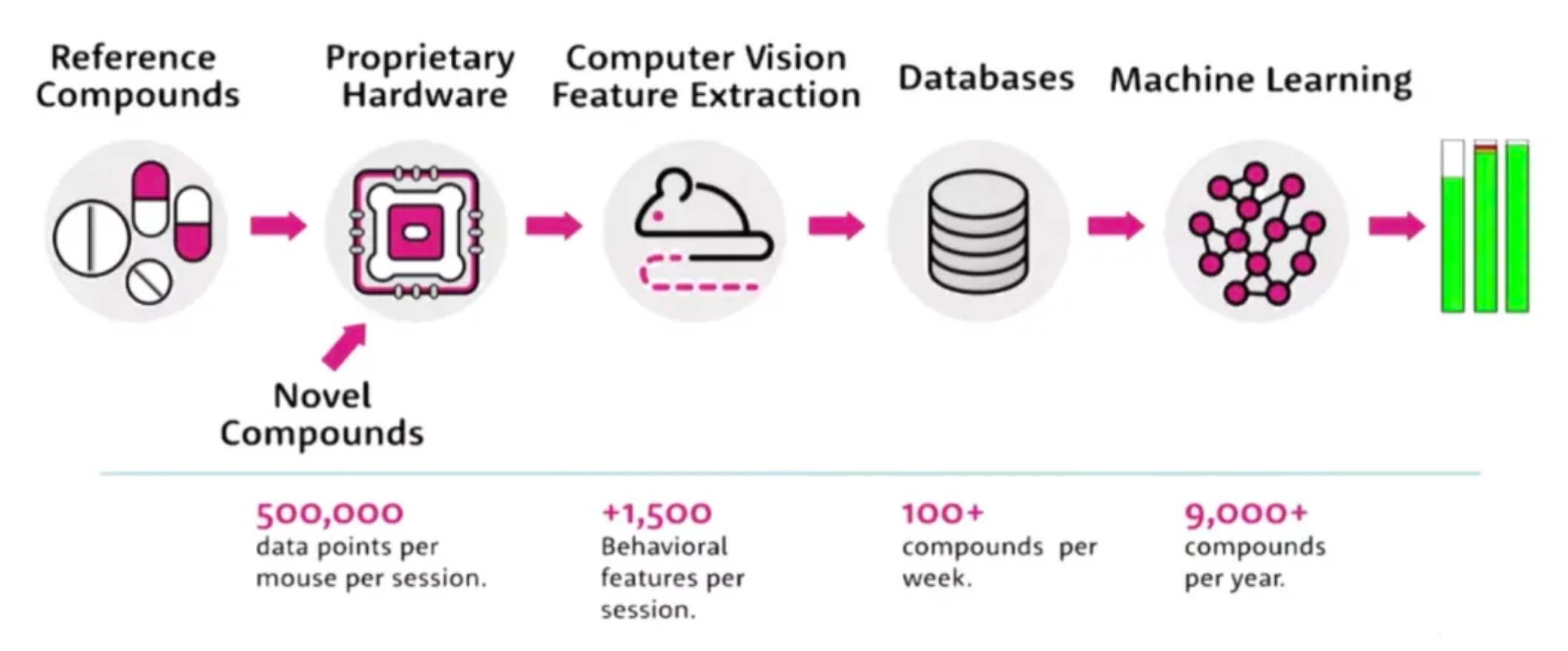
Compared to traditional behavioral testing methods, the SmartCube® system boasts the following advantages:
·High Automation: Using precise hardware devices, SmartCube® can automatically guide mice to participate in a variety of experimental challenges, reducing the errors associated with human interference and enhancing experiment efficiency and accuracy.
·Real-time Behavior Monitoring: Employing advanced computer vision technology, it captures and precisely identifies the mice's behavior in real-time, providing researchers with a wealth of behavioral data.
·AI Data Analysis: The built-in artificial intelligence algorithms are capable of deep learning and intelligent analysis on the vast amount of behavioral data collected, revealing behavioral patterns and physiological correlations that traditional methods may fail to detect.
·Multidimensional Assessment: Beyond basic behavioral parameters, SmartCube® also synchronously acquires physiological indicators, enabling comprehensive and multi-level evaluations.
·High Throughput Capacity: Compared to manual operation, SmartCube® can process large quantities of experimental samples more rapidly and efficiently, accelerating the process of drug screening and disease model research.
PsychoGenics has identified thousands of active behavioral compounds through the screening of various compound libraries, which can serve as starting points for new drug discovery projects. Typically, the discovery process begins with the search for "hits" that possess desired characteristics and other specific features. For instance, a project might focus on finding compounds with antidepressant features similar to certain psychedelic compounds but without activity on the 5HT2a receptor.
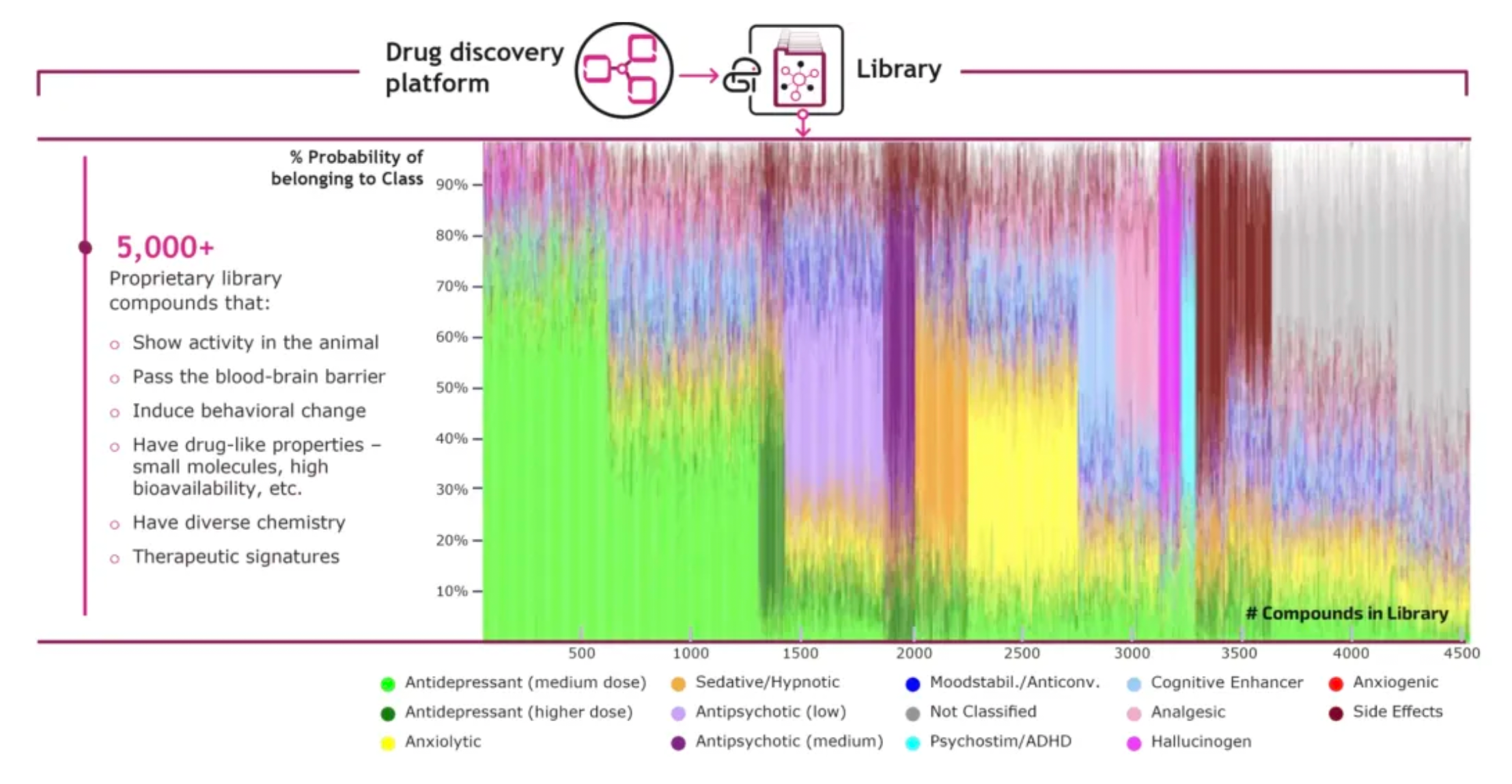
In preclinical studies, SEP-380135 has demonstrated a broad range of in vivo activity, preliminarily indicating its effectiveness against a variety of behavioral and psychological symptoms of dementia, including, but not limited to, agitation/aggression, psychomotor hyperactivity, and depressive symptoms. This suggests that SEP-380135 has the potential to be one of the effective medications for treating multiple complex symptoms associated with dementia, offering new possibilities for further improving the quality of life of patients with dementia.
SEP-4199 is a small molecule compound that functions as a dual antagonist targeting 5-HT receptors and D2 receptors. It is currently undergoing phase 3 clinical trials for the treatment of bipolar I disorder, major depressive disorder, and severe depression.
SEP-378614 is a small molecule compound jointly developed by Sumitomo and PsychoGenics, which is currently undergoing phase 1 clinical trials for the treatment of major depressive disorder.