Monoclonal vs. Bispecific Antibodies: Who Will Dominate Future Cancer Treatment?
Since the clinical introduction of Rituximab in 1997, monoclonal antibody (mAb) drugs have made significant progress in the field of cancer treatment due to their high specificity, high affinity, long half-life, and potent cytotoxic capabilities. These drugs have transformed various cancer treatment modalities by precisely targeting specific antigens on the surface of tumor cells.
In recent years, there has been notable advancement in the research and development of new drugs based on antibody targeting. In addition to traditional monoclonal antibodies, new types of drugs such as antibody-drug conjugates (ADCs) and bispecific antibodies have demonstrated great potential. For example, classic monoclonal antibodies like Rituximab and Trastuzumab have been unmatched in treating certain types of cancers, but their status is now being challenged with the emergence of these novel drugs.
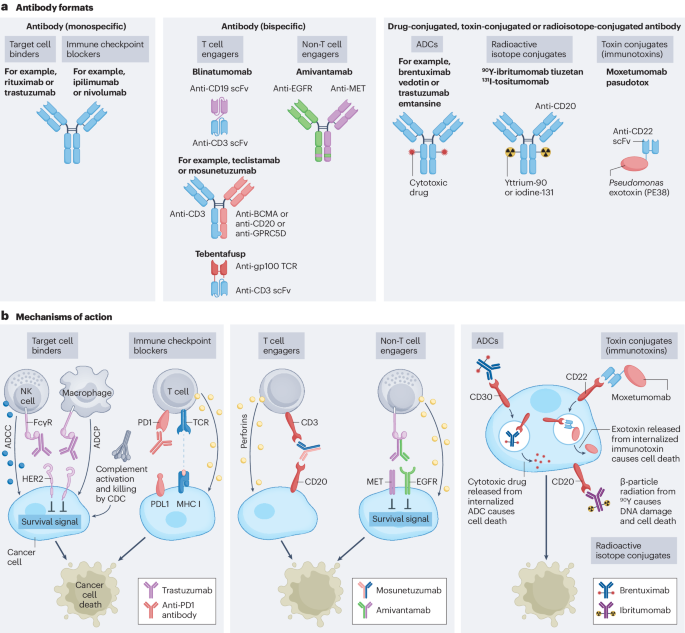
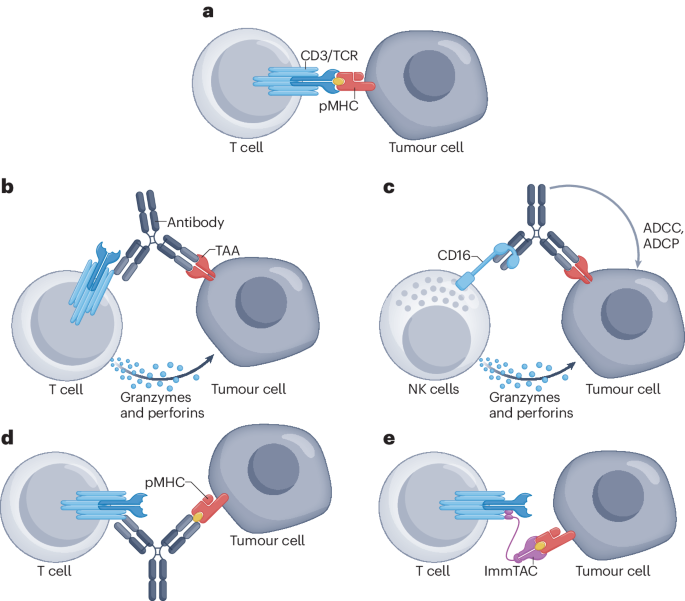
In the field of anti-tumor drugs, both monoclonal antibodies (mAbs) and bispecific antibodies (biAbs) are important therapeutic tools. Monoclonal antibodies work by specifically binding to antigens on the surface of tumor cells, blocking tumor growth signals or mobilizing the immune system to attack tumor cells. Bispecific antibodies, on the other hand, are capable of binding to two different antigens or two different epitopes of the same antigen, theoretically offering more diverse mechanisms of action, such as blocking two signaling pathways simultaneously or bringing immune cells closer to tumor cells to enhance the immune attack on the tumor.
The development and application of bispecific antibodies is becoming a new trend in cancer therapy. For instance, Akeso Biopharma has developed Cadonilimab (AK104), a PD-1/CTLA-4 bispecific antibody. It activates T cells and exerts an anti-tumor effect by simultaneously blocking the interaction between PD-1 and CTLA-4 and their ligands. Additionally, Kelun Botai has developed SKB571, a novel bispecific ADC (antibody-drug conjugate). This drug enhances cytotoxicity, overcomes resistance, and boosts tumor cell specificity to reduce side effects.
However, the development and production of bispecific antibody drugs face technological challenges, such as chain mispairing and complex structural design. Global pharmaceutical companies have developed over 50 core technology platforms to address these issues. With continuous technological advancements and strategic global deployments, bispecific antibody drugs have become an important option in cancer treatment.

In the development of new drugs, querying antibody sequences is crucial. Patsnap Bio provides a robust tool for retrieving and analyzing sequence information of antibodies. Through this database, researchers can access detailed sequence data on antibody drugs, including their core patent information, chemical modifications, and general sequence alignments. This information is essential for understanding the pharmacological mechanisms of the drugs, evaluating their therapeutic effects, and guiding further drug design and optimization.
Let's take the recently popular bispecific antibody Ivonescimab and the classic monospecific antibody Pembrolizumab as examples. Through Patsnap Bio, we can compare the characteristics of these two types of cancer drugs.
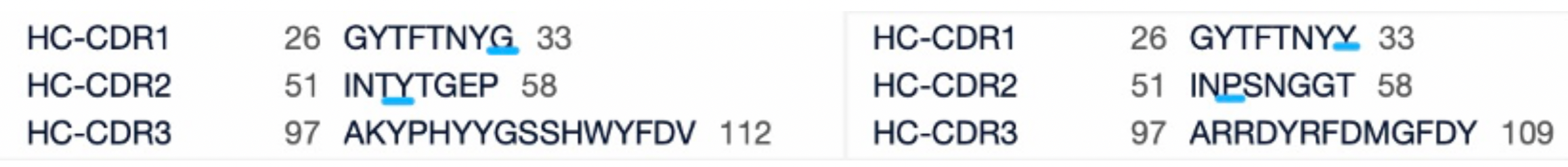
Ivonescimab and Pembrolizumab sequence comparison
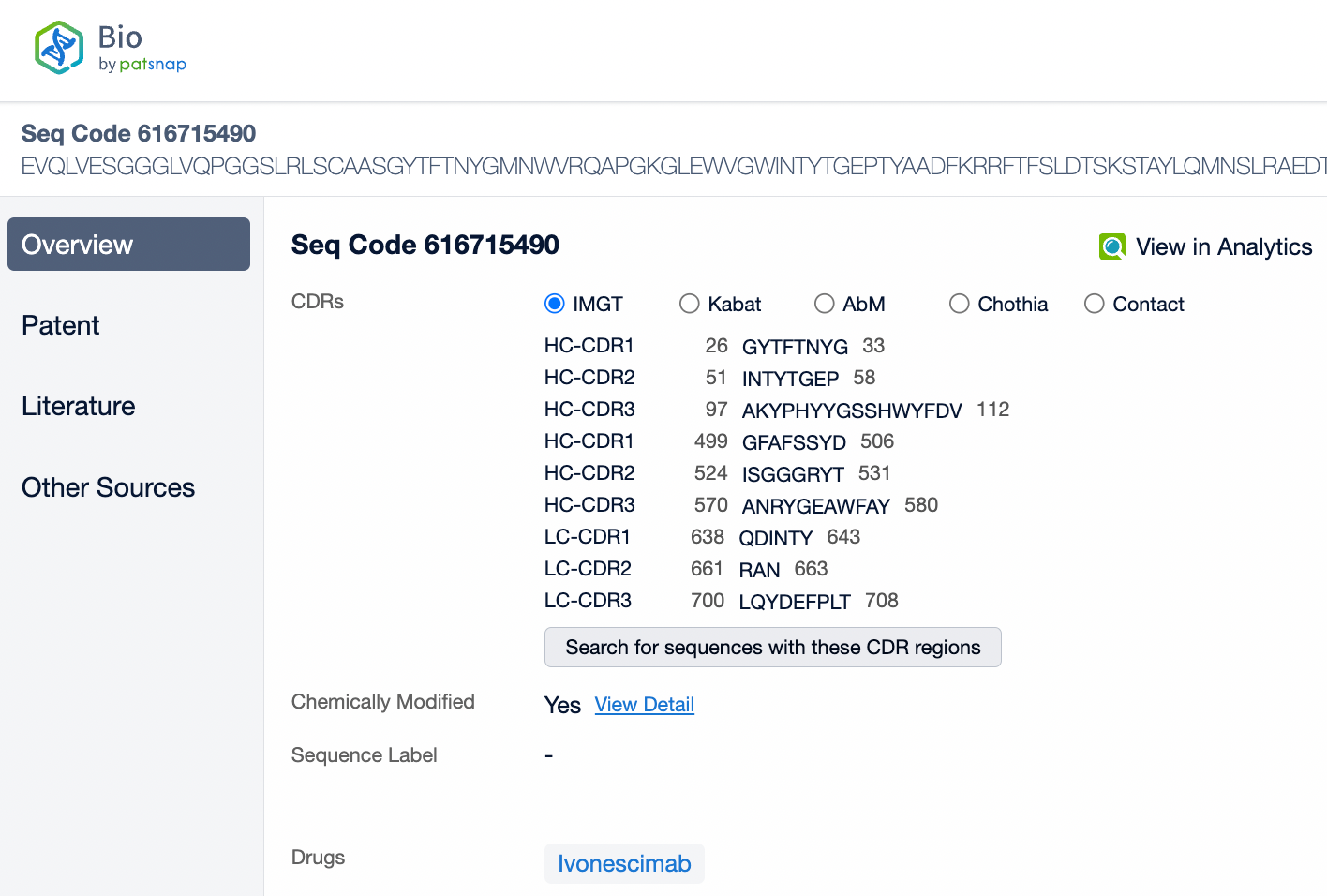
Ivonescimab is an innovative IgG1 subtype humanized bispecific antibody associated with strong antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). Its structural feature lies in its ability to simultaneously target two important cancer therapy targets: PD-1 and VEGF-A. The design of Ivonescimab allows it to block the binding of PD-1 to its ligand PD-L1, lifting the immunosuppression in the tumor microenvironment, and to block the binding of VEGF-A to its receptor VEGFR2, thereby inhibiting tumor angiogenesis. This dual mechanism of action helps to promote immune cell attacks on tumors and restricts the tumor's blood supply, jointly combating tumor growth. Ivonescimab's structure has been specially designed to make it a tetravalent antibody with two binding sites, each capable of independently binding with PD-1 and VEGF-A. Additionally, the Fc region of this antibody has been modified to reduce binding with Fcγ receptors on the surface of immune cells, helping to reduce potential immune-related side effects.
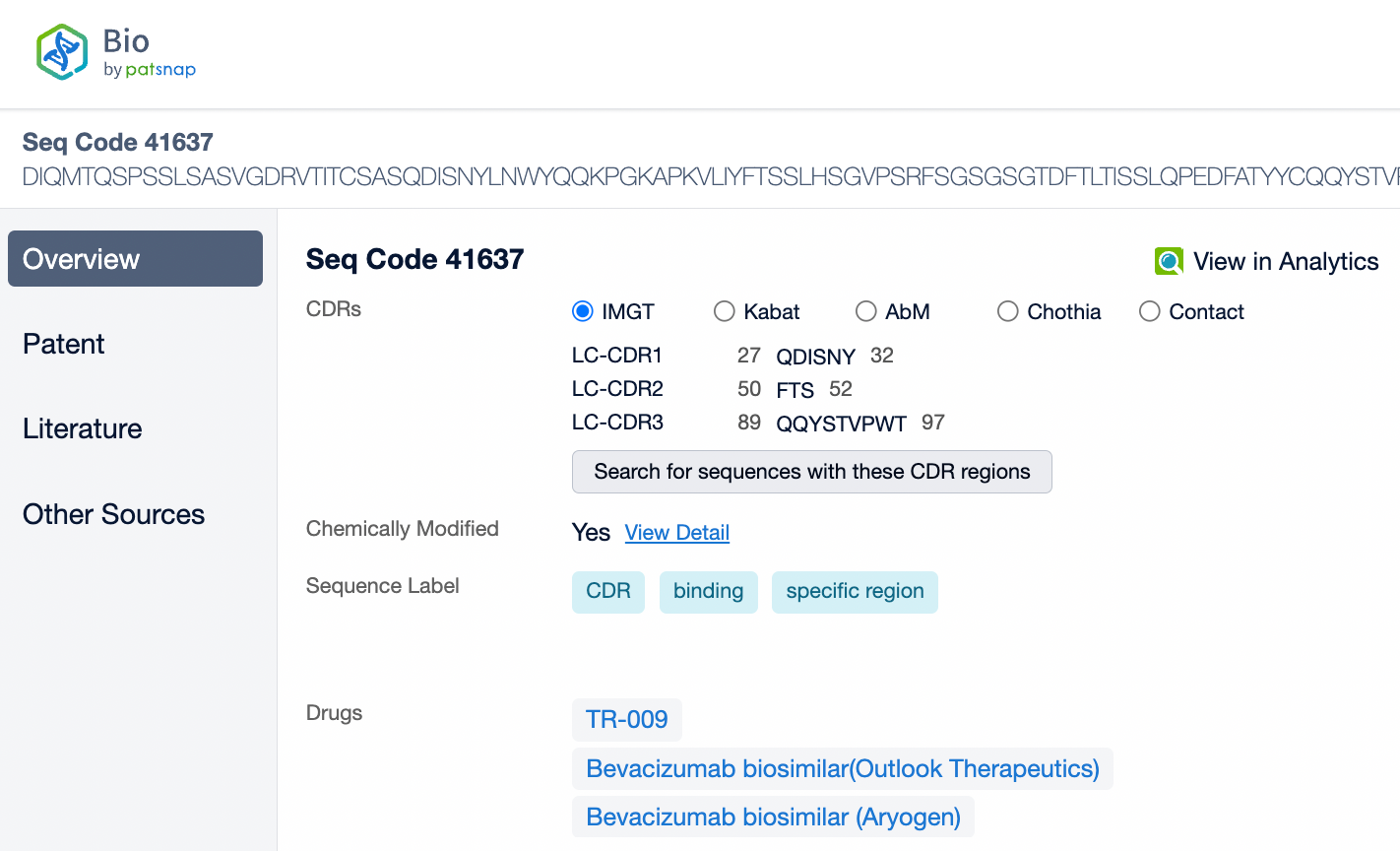
Pembrolizumab is a monoclonal antibody of the IgG4 subtype, characterized by low antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) effects. Pembrolizumab is composed of two light chains and two heavy chains, with each chain containing a variable region (V region) and a constant region (C region). In the C region of the heavy chain, the IgG4 subtype has a shorter hinge region, providing greater flexibility in vivo. The IgG4 subtype of pembrolizumab has undergone an S228P mutation, which helps prevent arm exchange in IgG4 antibodies, thereby maintaining the stability of the drug.
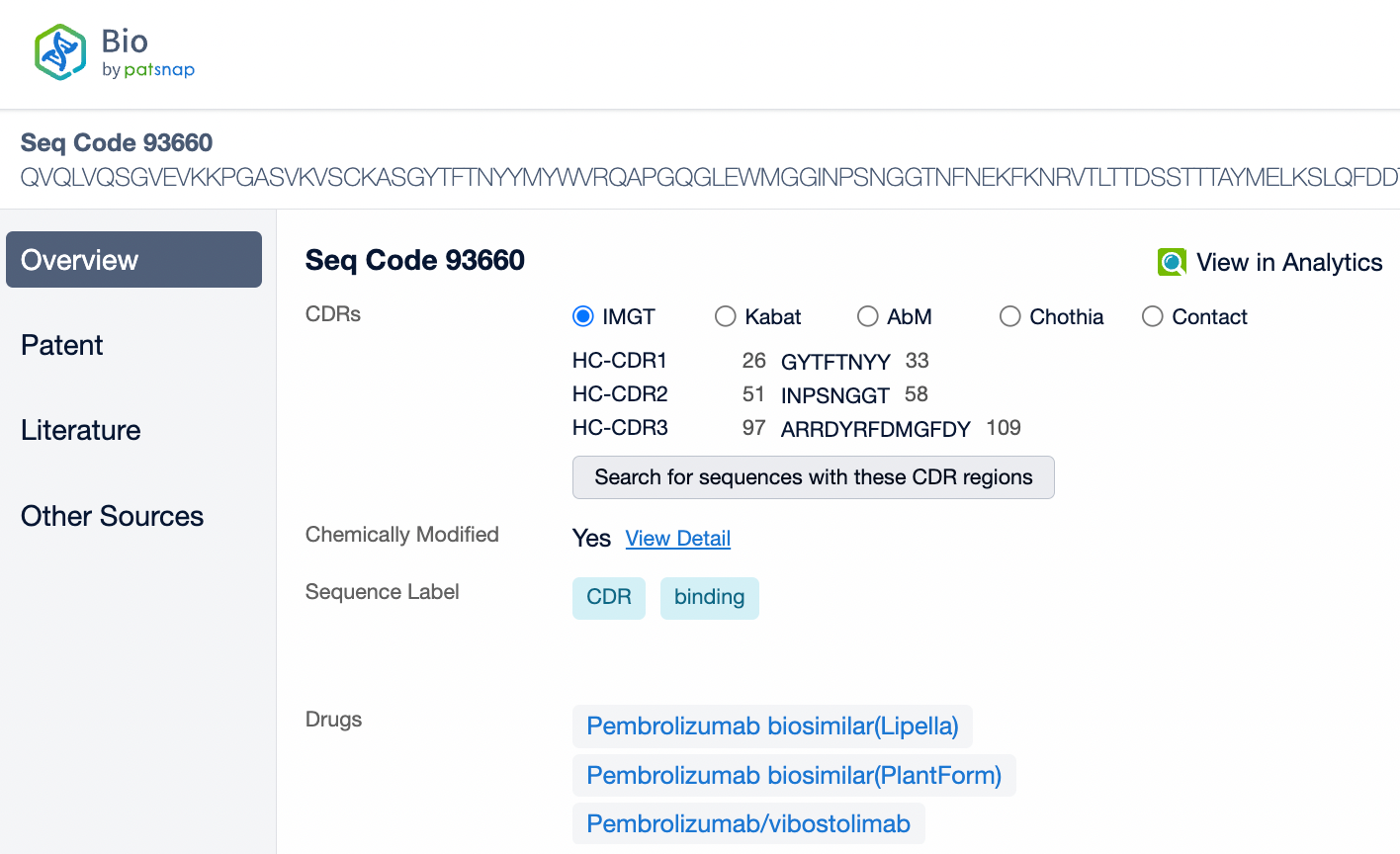
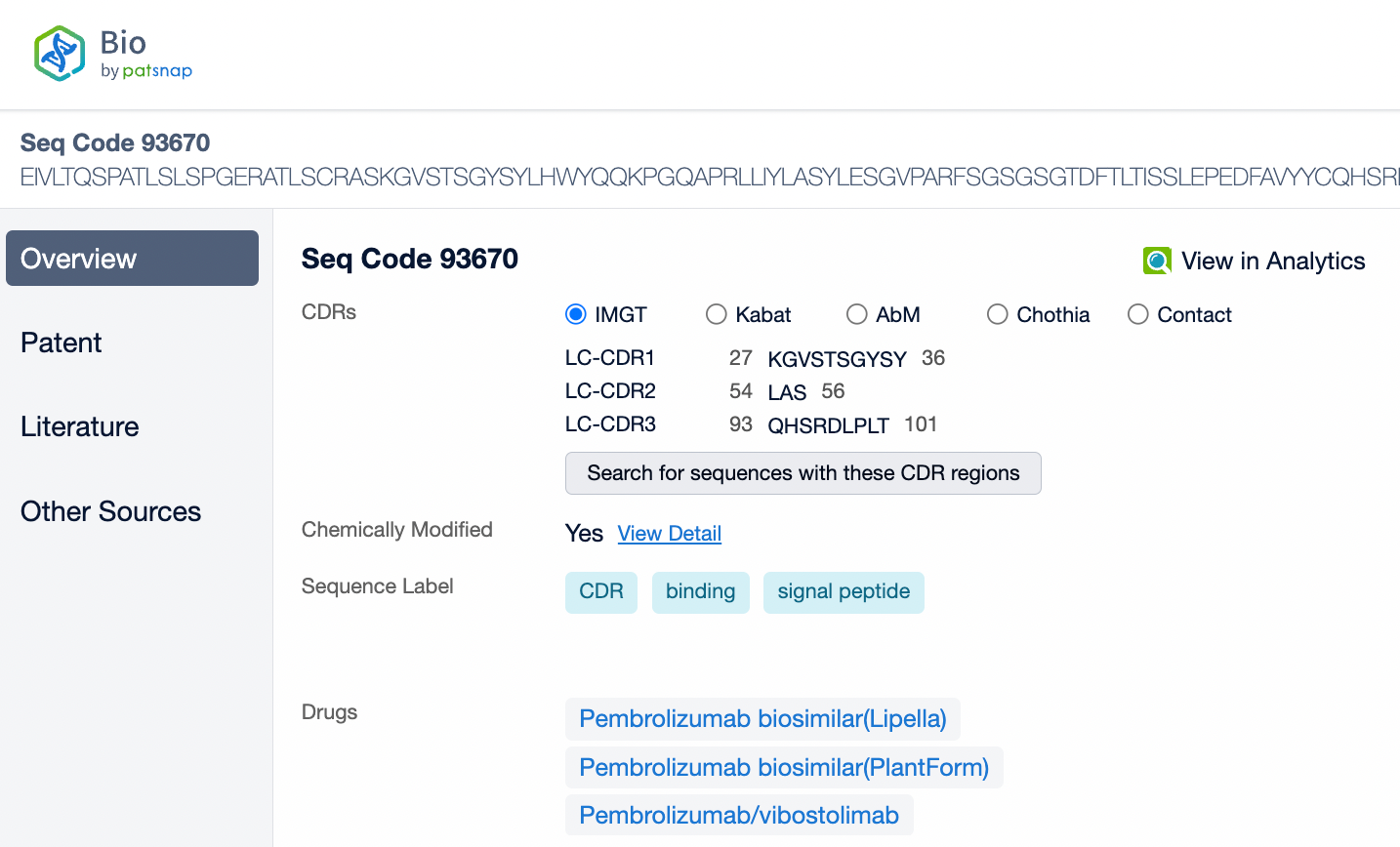
From the comparison of the complementarity-determining region (CDR) of monoclonal antibodies, it can be observed that Pembrolizumab and Ivonescimab exhibit a certain degree of sequence similarity. A query through Patsnap Bio revealed significant differences in the CDRs of the light chains between the two antibodies, whereas the differences in the heavy chain CDRs were smaller. This typically reflects the manner in which antibodies recognize antigen specificities. Both the light chain and heavy chain CDRs jointly determine the antibody's affinity and specificity for antigens, with CDRH3 and CDRL3 being particularly crucial as they often exhibit the highest sequence diversity and play a key role in antibody-antigen binding. The larger differences in the light chain CDRs suggest that the light chains play a more flexible role in forming the unique three-dimensional structure of the antibodies, which is essential for recognizing different types of antigens. In contrast, the relatively small variations in the heavy chain CDRs may indicate that the heavy chains provide a more conservative recognition pattern in these two antibodies, facilitating highly specific antigen recognition.

In medical reviews and patent approval processes, the significance of Complementarity-Determining Regions (CDRs) is particularly important. CDRs are key to the uniqueness of an antibody, directly related to the functional properties of the antibody, including its affinity and specificity for the target antigen. Therefore, during patent approvals, CDR sequences are often used to determine whether an invention is novel and inventive, and whether it differs from existing technology. If a newly developed antibody has significant differences in its CDR sequences compared to an antibody protected by an existing patent, it may be considered an independent new invention, thereby obtaining patent protection.
In the process of medical review, regulatory authorities also carefully examine the CDR sequences to confirm the safety, efficacy, and uniqueness of the antibody drug. This means that CDR sequences are not only a key factor in assessing how an antibody drug differs from known products, but also vital in determining whether it meets unmet medical needs. In summary, CDR sequences play a crucial role in the research and development, patent protection, and regulatory approval of antibody drugs.
Since the first clinical application of Rituximab in 1997, monoclonal antibodies have forged new paths in cancer treatment due to their high specificity, strong affinity, long half-life, and powerful antitumor effects, completely altering treatment standards for various cancers, including Non-Hodgkin's Lymphoma. However, with the rapid development of biopharmaceutical technology, monoclonal antibody drugs now face competition from new therapeutic modalities such as bispecific antibodies (BsAbs). BsAbs, with their unique dual-targeting capability, have shown unprecedented potential in simultaneously blocking multiple signaling pathways or promoting the interaction between immune and tumor cells. This not only broadens the strategic range of tumor treatments but also provides new solutions to the issues of resistance and limited efficacy encountered in monoclonal antibody therapies.
In choosing between monoclonal and bispecific antibodies, each has its distinct features: monoclonal antibodies, through years of research and practice, have established an irreplaceable position in the treatment of various cancers, particularly where targeting a single antigen effectively controls the disease. Bispecific antibodies, with their unique mechanism of acting on two different antigens or two different epitopes on the same antigen, represent a new hope for tackling complex diseases and enhancing treatment outcomes. From a market perspective, the development of bispecific antibodies is increasingly heating up. Numerous biopharmaceutical companies are investing substantial resources to develop these drugs, aiming to capture a share of this emerging market. Nevertheless, the development of bispecific antibodies also faces many technical challenges, including the complexity of production processes, in vivo stability, and pharmacokinetics. Despite these challenges, the multiple advantages of bispecific antibodies in treatment indicate their vast potential in the future of cancer immunotherapy, possibly leading to a new revolution in the pharmaceutical industry.
Better answers for better bio-innovations!
Validate novelty, eliminate risk, and innovate with confidence using the world’s largest sequence database curated from millions of patent and non-patent sources.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.
With best-in-class coverage of protein and nucleic acid sequences combined with state-of- the-art search algorithms, you’ll spend less time searching and more time bringing your bio-innovations to market.
Reference
1.Paul, S. et al. Cancer therapy with antibodies. Nat Rev Cancer 24, 399-426 (2024). https://s10-doi-org.libproxy1.nus.edu.sg/s41568-024-00690-x
2.Goebeler, M. E., Stuhler, G. & Bargou, R. Bispecific and multispecific antibodies in oncology: opportunities and challenges. Nat Rev Clin Oncol 21, 539-560 (2024). https://s10-doi-org.libproxy1.nus.edu.sg/s41571-024-00905-y