TILT Biotherapeutics Reveals TILT-123 Clinical Results at ESMO 2024
TILT Biotherapeutics (TILT), a biotechnology firm at the clinical stage focused on cancer immunotherapies, has declared its intention to showcase data at the European Society of Medical Oncology (ESMO) Congress 2024. The findings continue to illustrate the promise of TILT-123 as an intravenous treatment.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The data summarized highlights the outcomes from a Phase I clinical trial cohort examining TILT-123 monotherapy for advanced solid tumors (NCT04695327). The findings indicate that this treatment is safe, induces tumor transduction, and elicits immunological responses in metastatic lesions. A study involved six patients with different cancer types: three with rectal carcinoma, one with gastric intestinal type carcinoma, one with pancreatic ductal adenocarcinoma, and one with liposarcoma. TILT-123 was administered bi-daily initially twice a week, then every three weeks. Among patients assessable by imaging by the data cutoff point, disease control was observed in 33% according to RECIST1.1 and in 66% using PET-based criteria. Updated results will be disclosed at the meeting (1).
TILT Biotherapeutics’ founder and CEO, Akseli Hemminki, a cancer specialist with extensive experience in treating patients with oncolytic viruses, commented, “The data presented at ESMO 2024 further corroborates the potential of TILT-123 as a fully intravenous therapy for challenging cancer cases. Additionally, we are making significant headway in our two other studies investigating this fully intravenous regimen of TILT-123. Our trajectory towards Phase II is on track, and we are achieving promising clinical advancements.”
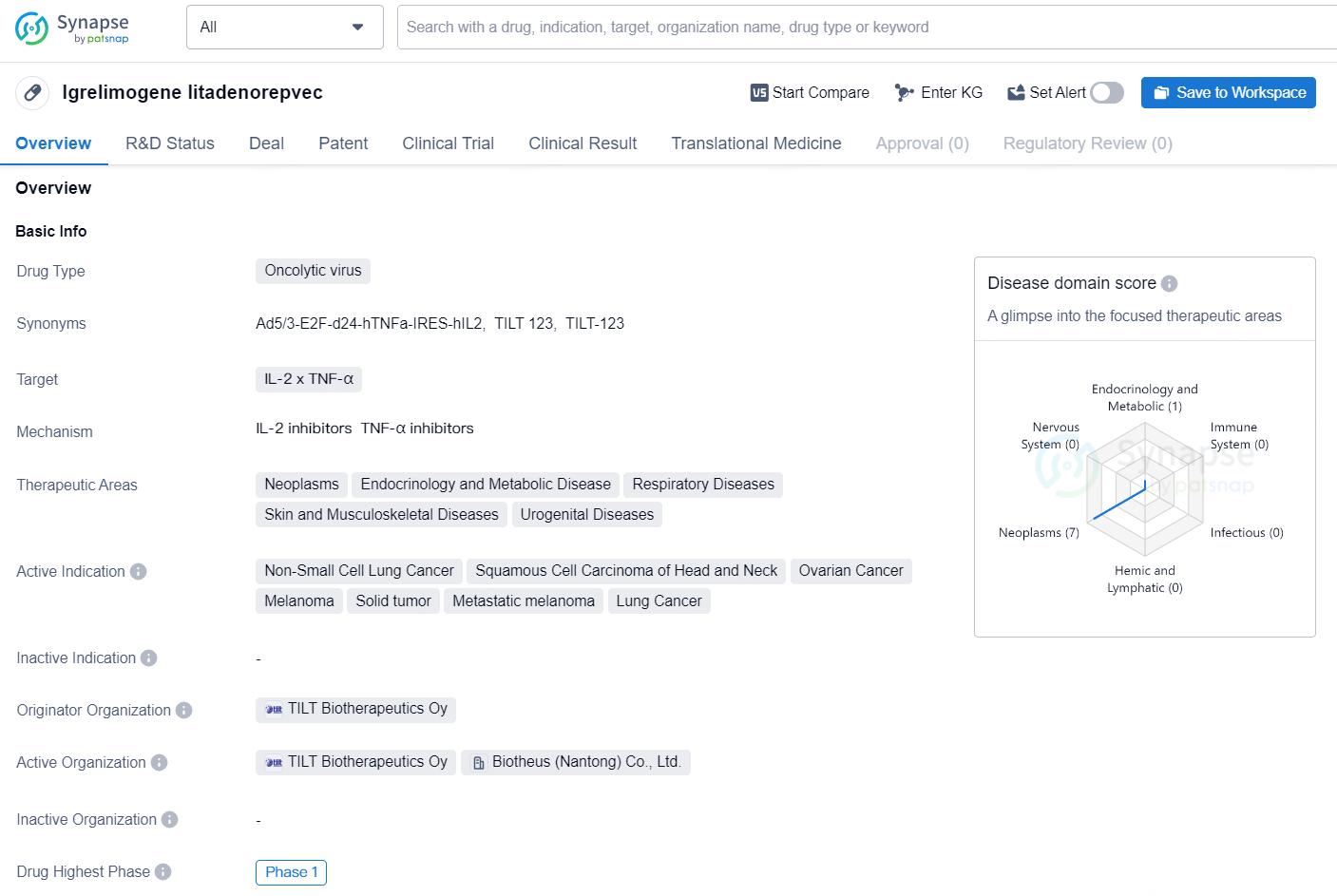
TILT-123 is an oncolytic adenovirus engineered with tumor necrosis factor alpha (TNFα) and interleukin-2 (IL-2) to boost the effectiveness of T-cell therapies, encompassing immune checkpoint blockade and adoptive cell transfer. TILT's strategy involves using oncolytic viruses to selectively reproduce within and destroy cancer cells, while also enhancing anti-tumor immune responses.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
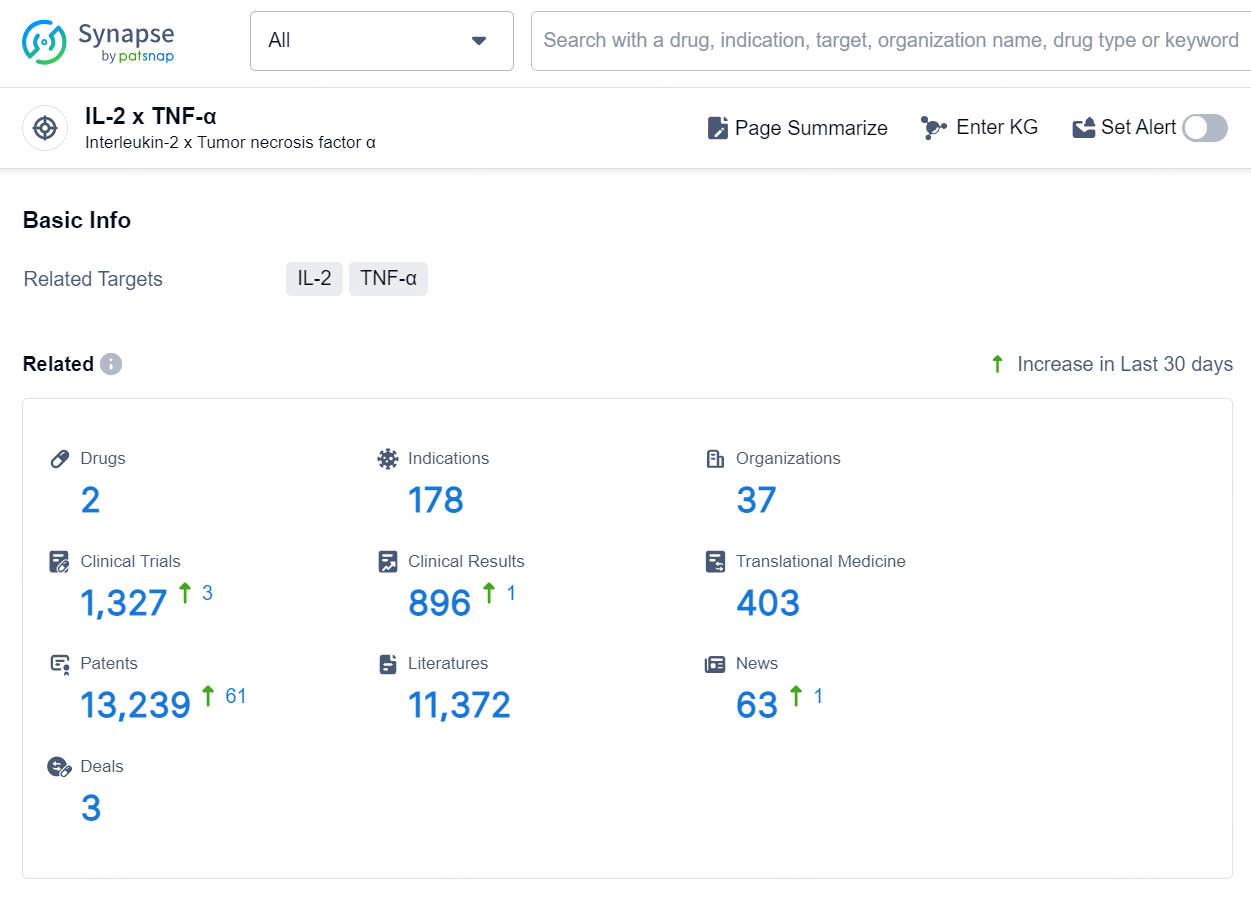
According to the data provided by the Synapse Database, As of September 11, 2024, there are 2 investigational drug for the IL-2 x TNF-α targets, including 178 indications, 37 R&D institutions involved, with related clinical trials reaching 1327, and as many as 13239 patents.
Lgrelimogene litadenorepvec is an oncolytic virus drug that targets IL-2 x TNF-α and is being developed for various therapeutic areas including neoplasms, endocrinology and metabolic disease, respiratory diseases, skin and musculoskeletal diseases, and urogenital diseases. The drug is actively being investigated for the treatment of non-small cell lung cancer, squamous cell carcinoma of the head and neck, ovarian cancer, melanoma, solid tumor, metastatic melanoma, and lung cancer.