Monte Rosa Therapeutics Announces Initial Participants Dosed in First Phase of MRT-6160 Study
Monte Rosa Therapeutics, Inc. (Nasdaq: GLUE), a biotechnology firm in the clinical stage concentrating on innovative molecular glue degrader (MGD)-based treatments, has announced that it has administered doses to the first participants in a Phase 1, single ascending dose/multiple ascending dose (SAD/MAD) study involving healthy volunteers. This trial is evaluating MRT-6160, an MGD aimed at VAV1, intended for treating systemic and neurological autoimmune conditions. The Company anticipates initial results from this Phase 1 trial by the first quarter of 2025.
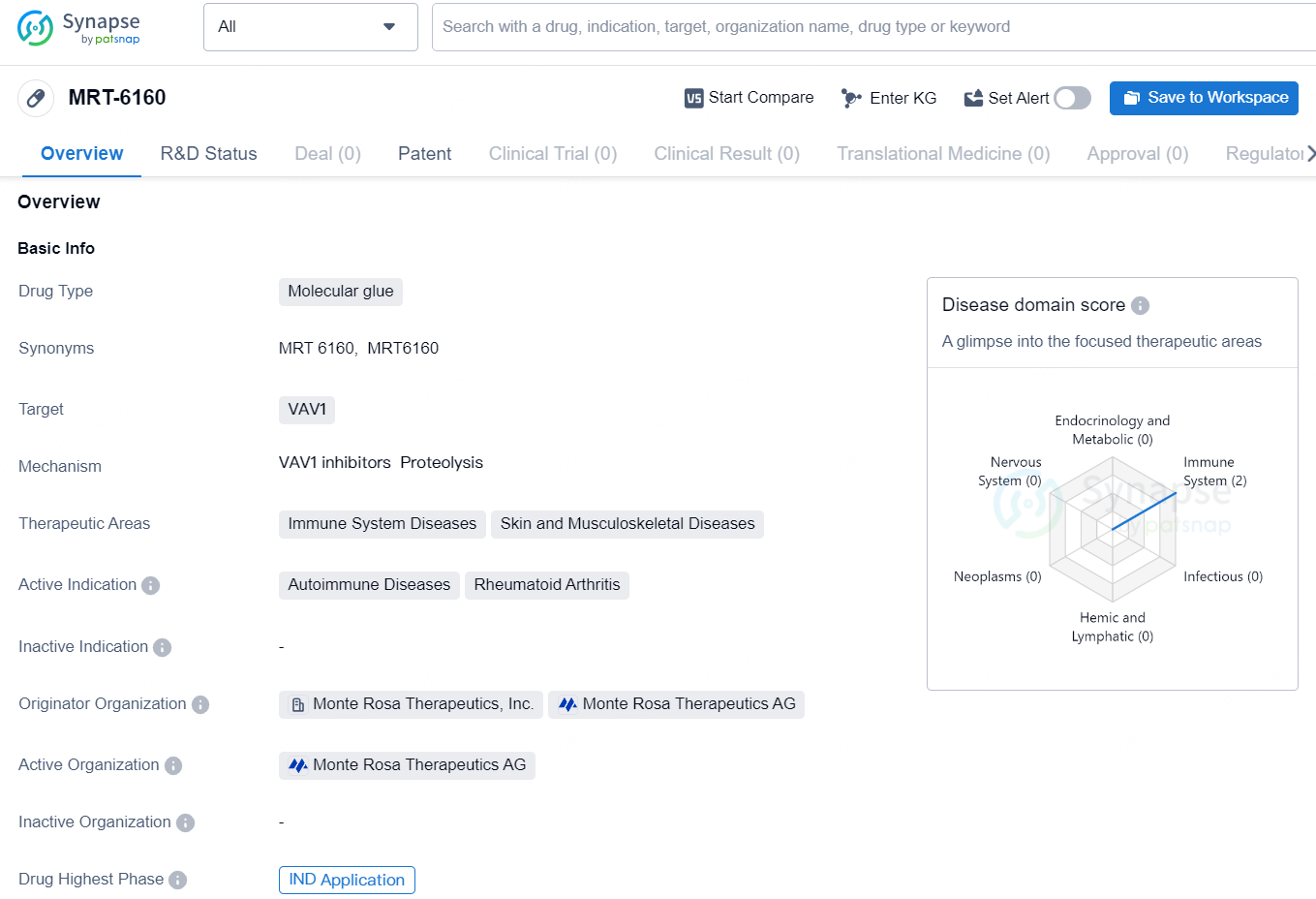
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"We are thrilled to commence our Phase 1 clinical trial of MRT-6160, a highly selective, potent, and orally bioavailable VAV1-focused MGD, which we believe is the inaugural rationally designed MGD targeting a non-oncology indication," stated Markus Warmuth, M.D., Chief Executive Officer of Monte Rosa Therapeutics.
"Our MGD-based therapeutic strategy is adept at targeting proteins that conventional methods struggle to address. We see promising opportunities to apply our technology to well-defined targets such as VAV1, which were previously deemed undruggable. By degrading VAV1, a crucial regulator of T- and B-cell receptor activities, MRT-6160 may provide a unique approach to treating various autoimmune and inflammatory diseases. The Phase 1 trial of MRT-6160 is structured to gather early data on safety, pharmacokinetics, VAV1 protein degradation, and essential downstream pharmacodynamic markers such as CD69, IL-2, IL-6, and IL-17. This data will guide our clinical strategy further. We are eager to share the initial clinical findings in Q1 2025 and to subsequently launch anticipated proof-of-concept studies in ulcerative colitis, rheumatoid arthritis, and possibly other conditions."
The progression of MRT-6160 is supported by preclinical data in various models of autoimmune/inflammatory diseases and preclinical GLP toxicology data, suggesting a unique therapeutic profile for T-cell, T/B-cell, and Th17-mediated systemic and neurological autoimmune diseases. MRT-6160 has demonstrated potent and selective degradation of VAV1 in vitro in human T and B cells and shown promising outcomes in multiple preclinical studies of autoimmune diseases, including inflammatory bowel disease, rheumatoid arthritis, and multiple sclerosis models.
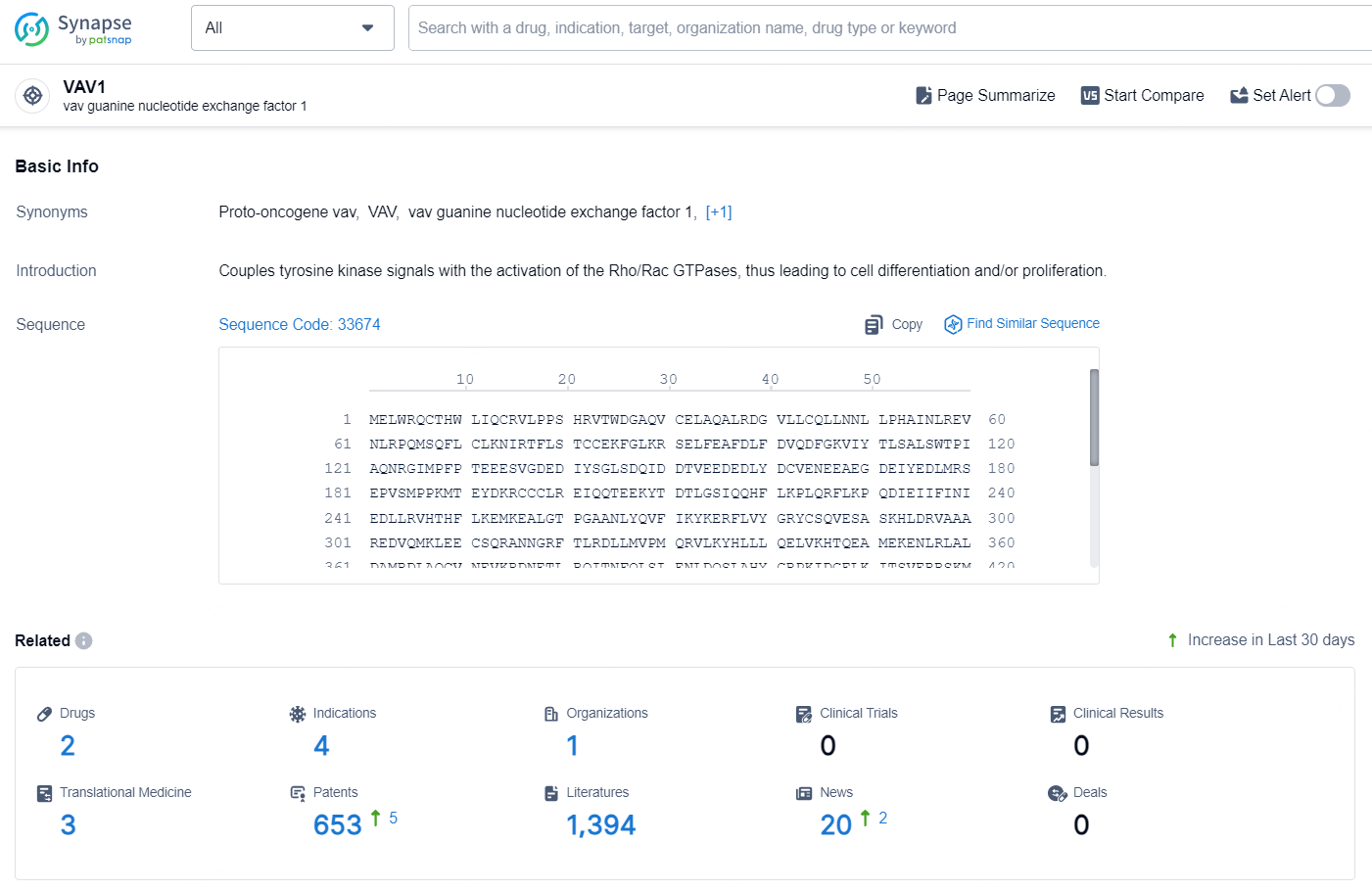
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 21, 2024, there are 2 investigational drugs for the VAV1 target, including 4 indications, 1 R&D institution involved, and as many as 653 patents.
MRT-6160 is a potent, highly selective, and orally bioavailable investigational molecular glue degrader of VAV1, which in preclinical studies has shown deep degradation of its target with no detectable effects on other proteins. VAV1, a Rho-family guanine nucleotide exchange factor, is a key signaling protein downstream of both the T- and B-cell receptors. VAV1 expression is restricted to blood and immune cells, including T and B cells. Preclinical studies have shown that targeted degradation of VAV1 protein via an MGD modulates both T- and B-cell receptor-mediated activity. This modulation is evident both in vitro and in vivo, demonstrated by a significant decrease in cytokine secretion, proteins vital for maintaining autoimmune diseases.