Monte Rosa Therapeutics reveals preclinical results, suggesting that MRT-6160, could treat immune-based and inflammatory disorders
Monte Rosa Therapeutics, Inc., a firm in the clinical-stage biotechnology sector that produces medicines based on novel molecular glue degrader (MGD), has disclosed that it will share initial experimental results at the American College of Rheumatology Convergence Annual Meeting taking place in San Diego, CA, between November 10-15. The findings reveal that MRT-6160, an innovative, highly selective MGD that targets VAV1, had a mitigating effect on disease advancement in a mouse model of collagen-induced arthritis.
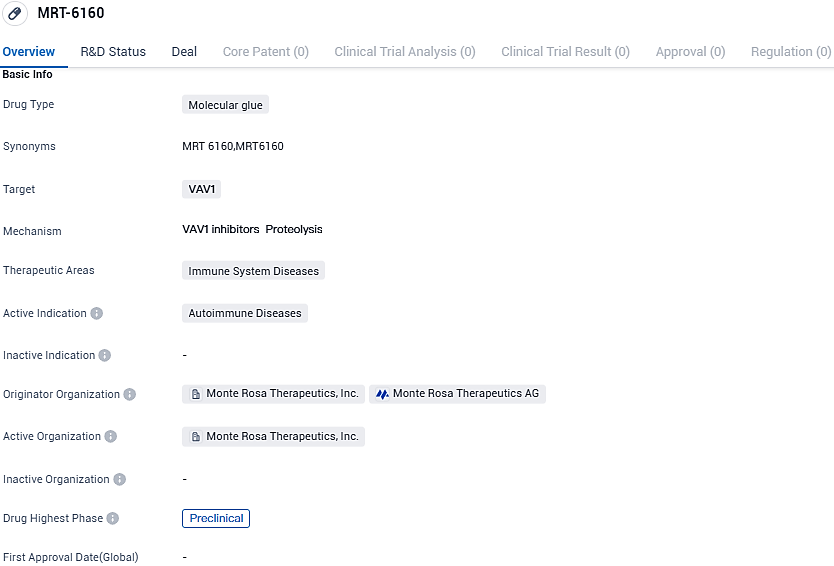
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
During lab tests, MRT-6160 demonstrated the ability to selectively degrade VAV1 and suppress the activation and functioning of primary human T-cells and B-cells prompted by TCR and BCR. In the collagen-induced arthritis (CIA) model, the oral administration of MRT-6160 resulted in swift VAV1 breakdown in numerous tissues, in accordance with the dosage.
Throughout a 20-day period, MRT-6160 noticeably reduced the progress of the disease and final functional grades compared to a control vehicle. Also, it pointed towards outstanding performance compared to an anti-TNF antibody.
“We're greatly inspired by these findings from our early-stage trials as we think they underscore the significance of VAV1 as a possible therapeutic target for T- and B-cell mediated autoimmune disorders.
They also point to MRT-6160's potential for wide-spread treatment of diseases caused by autoimmune and inflammation including rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, psoriasis and other autoimmune diseases," expressed Dr. Owen Wallace, the Chief Science Officer at Monte Rosa Therapeutics.
Dr. Wallace added, “Combined with comprehensive data from other autoimmune model trials, these encouraging outcomes solidify the position of MRT-6160 as it moves towards clinical stages. We are eager for our expected IND submission in the first half of the forthcoming year.”
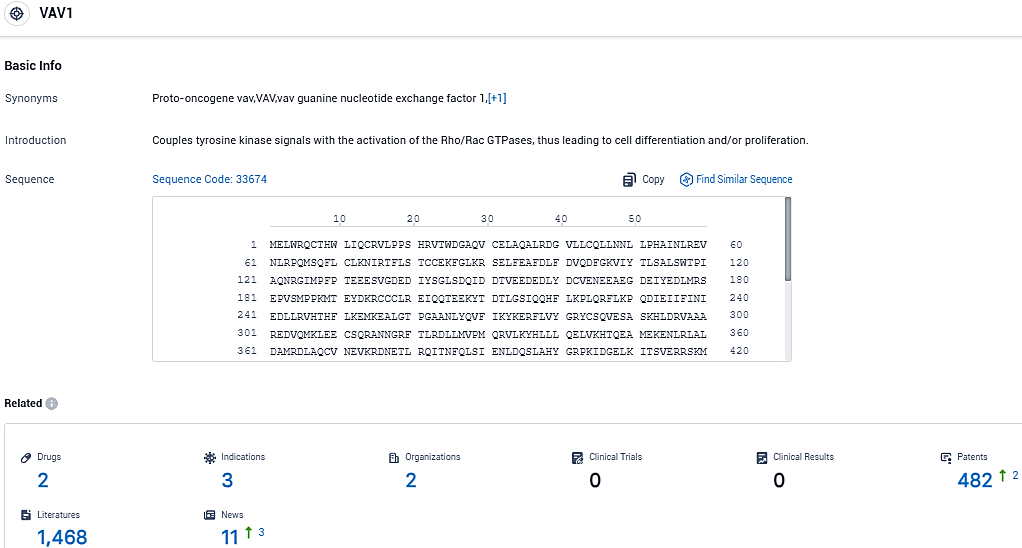
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 14, 2023, there are 2 investigational drugs for the VAV1 target, including 3 indications, 2 R&D institutions involved,and as many as 1468 patents.
MRT-6160 is a potent, highly selective, and orally bioavailable degrader of VAV1, which has shown deep degradation of its target with no detectable effects on other proteins. Preclinical studies demonstrate MRT-6160 inhibits disease progression in in vivo autoimmunity models.