Sunvozertinib: brief review of its R&D progress and the clinical outcome in 2023 ESMO
Sunvozertinib (DZD9008) is a rationally designed, irreversible EGFR inhibitor targeting EGFR mutations with wild-type EGFR selectivity. And the preliminary results of sunvozertinib in treatment naïve EGFR exon20ins NSCLC were reported at the ESMO Congress.
Sunvozertinib's R&D Progress
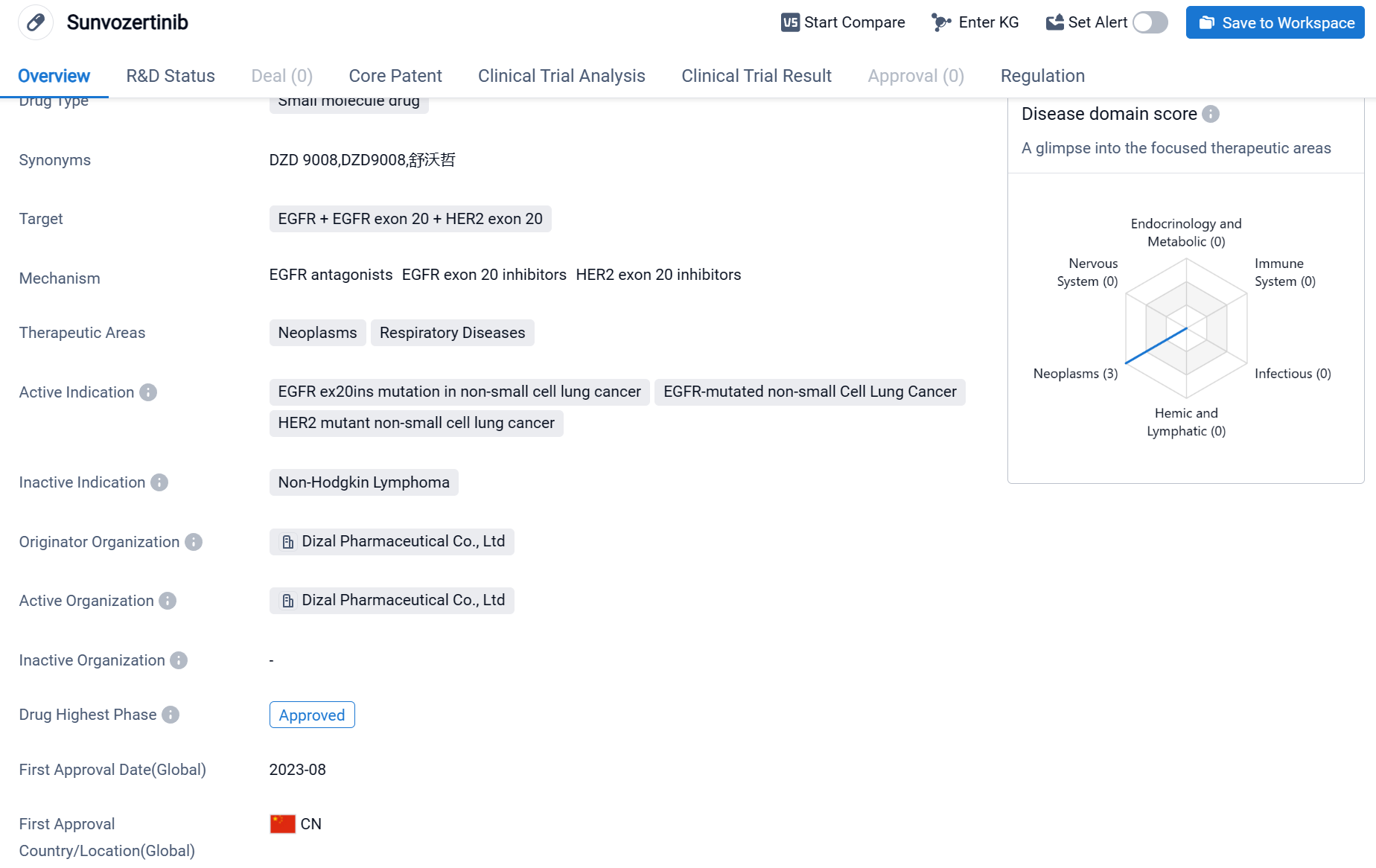
Sunvozertinib is a small molecule drug that falls under the category of biomedicine. It targets EGFR (Epidermal Growth Factor Receptor), EGFR exon 20, and HER2 exon 20. The drug is primarily used in the treatment of neoplasms and respiratory diseases. Sunvozertinib is specifically indicated for the treatment of EGFR ex20ins mutation in non-small cell lung cancer, EGFR-mutated non-small cell lung cancer, and HER2 mutant non-small cell lung cancer.
According to the Patsnap Synapse, Sunvozertinib has received approval for use in both global markets. And the clinical trial areas for Sunvozertinib are primarily in the United States, China and France. The key indication is EGFR-mutated non-small Cell Lung Cancer. 
Detailed Clinical Result of Sunvozertinib
The randomized, parallel assignment, open-labeled clinical trial (NCT05668988) was aimed to evacuate the efficacy and safety of sunvozertinib in NSCLC patients with EGFR Exon20 insertion mutations.

In this study, NSCLC patients with EGFR exon20ins who did not receive prior systemic anti-cancer therapy were enrolled into two ongoing studies: WU-KONG1, a multinational phase I/II study; and WU-KONG15 (NCT05559645), a phase II investigator-initiated study. Sunvozertinib was administered orally at 200 mg or 300 mg QD until discontinuation criteria were met. Efficacy analysis included patients with at least one post-treatment tumor assessment according to RECIST 1.1 by independent review committee (IRC). 
The result showed that as of February 21, 2023, a total of 28 patients were included in the efficacy analysis (200 mg, n = 19; 300 mg, n = 9). Median age was 67 years, and 75.0% (21/28) were female. Baseline ECOG PS was 0 or 1. Majority of patients (96.4%, 27/28) had metastatic diseases at study entry, with 21.4% (6/28) having > 3 metastatic sites and 32.1% (9/28) having baseline brain metastasis (BM). The most frequent mutation subtypes included 769_ASV (39.3%, 11/28), 770_SVD (10.7%, 3/28) and others. By IRC, 20 patients achieved tumor response, with a best objective response rate (ORR) of 71.4% (200 mg, 68.4%; 300 mg, 77.8%). Median duration of response has not been reached. Safety findings were consistent with results of previous sunvozertinib studies. The most common TEAEs included diarrhea, CPK increase, and skin rash. Majority of the AEs were of CTCAE grade 1 or 2, and clinically manageable.
It can be concluded that in the 1st line setting, sunvozertinib as monotherapy demonstrated promising anti-tumor efficacy and acceptable safety profile in NSCLC patients with EGFR exon20ins. A phase III, multinational, randomized study (WU-KONG28, NCT05668988) is ongoing to compare sunvozertinib to chemotherapy as 1st line treatment for EGFR exon20ins NSCLC.
How to Easily View the Clinical Results Using Synapse Database?
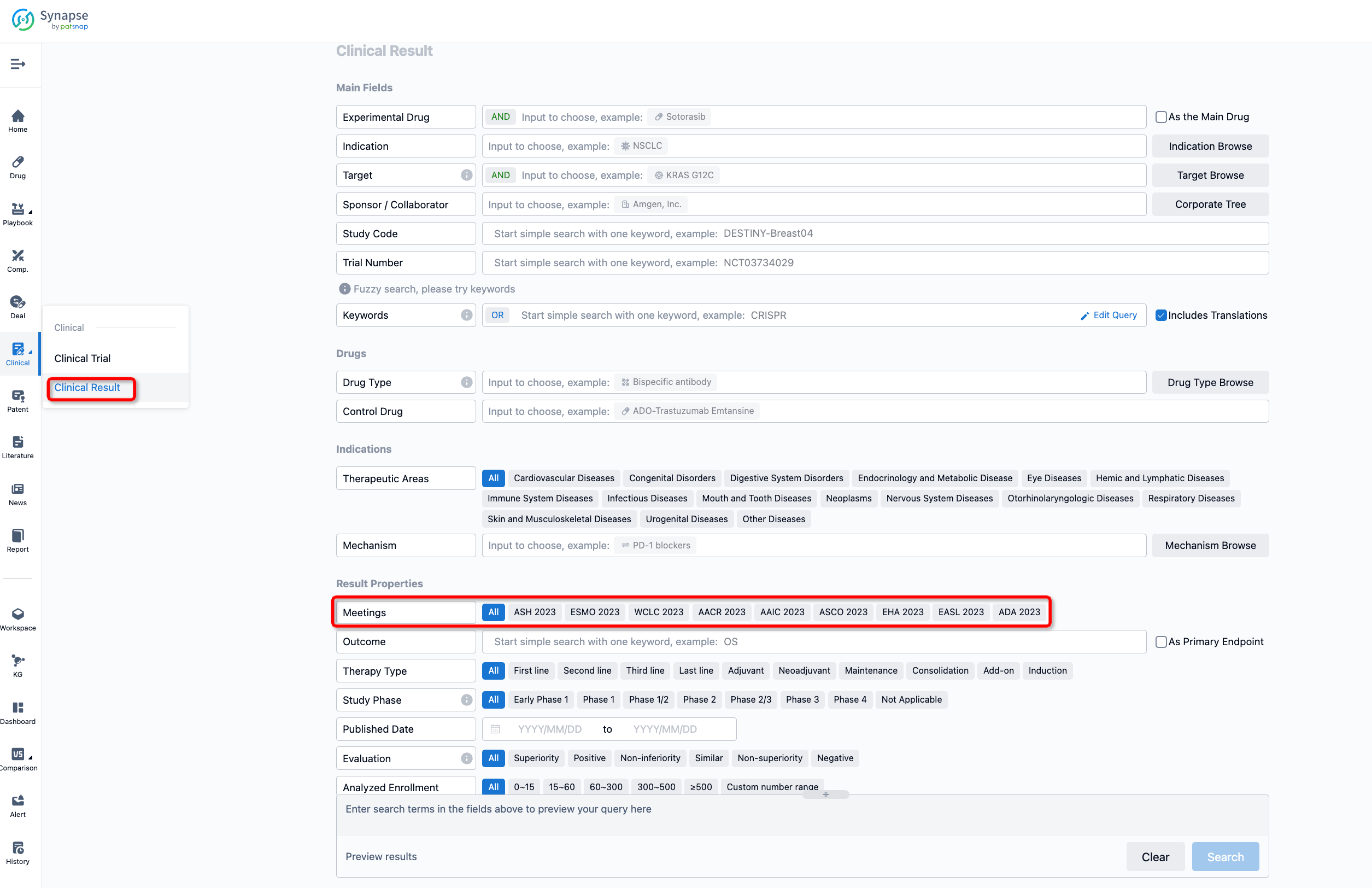
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.


Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!