Nature Communications Reveals Key Data on CSL and Arcturus COVID-19 Vaccine Efficacy and Safety
Global biotechnology leader CSL, along with Arcturus Therapeutics, has revealed that Nature Communications has published findings from a comprehensive phase 1/2/3a/3b study. This study assessed the safety, immunogenicity, and efficacy of ARCT-154, a pioneering self-amplifying COVID-19 vaccine, which is also the first approved sa-mRNA COVID-19 vaccine globally.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The findings indicate that two doses of 5 μg of the ARCT-154 sa-mRNA vaccine were well-received, elicited an immune response, and offered significant protection against various strains of COVID-19. The vaccine demonstrated 100 percent efficacy against severe COVID-19 in healthy individuals aged 18-59 and over 90 percent efficacy in individuals at higher risk of severe disease outcomes due to comorbidities or advanced age.
“These findings published in Nature Communications showcase ARCT-154's effectiveness and tolerability, contributing to the mounting evidence that our sa-mRNA vaccine holds significant promise in providing essential protection against the widespread virus, thus supporting our commitment to public health,” stated Jon Edelman, M.D., Senior Vice President, Vaccines Innovation Unit, CSL.
“We are delighted that the ARCT-154 study results have appeared in the esteemed journal Nature Communications,” commented Pad Chivukula, Ph.D., Chief Scientific Officer of Arcturus Therapeutics. “These data, alongside Japan's approval, underscore our and CSL's dedication to offering cutting-edge technology to safeguard the public from COVID-19.”
The article in Nature Communications, titled “Safety, immunogenicity, and efficacy of the self-amplifying mRNA ARCT-154 COVID-19 vaccine: pooled phase 1, 2, 3a, and 3b randomized, controlled trials,” was made available online.
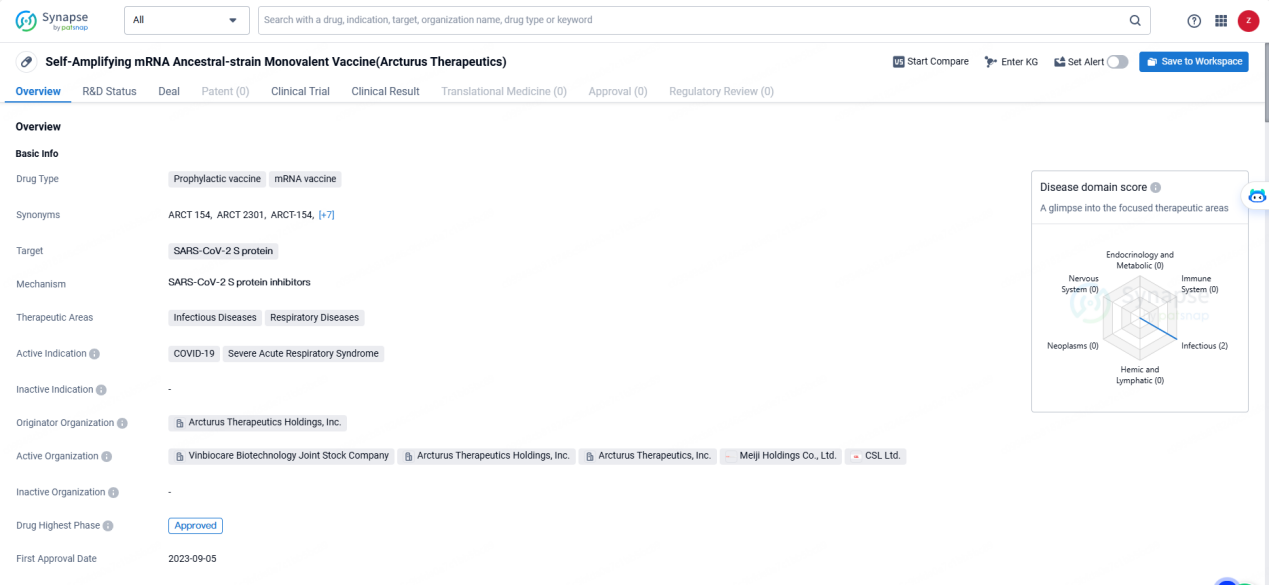
The Self-Amplifying mRNA Ancestral-strain Monovalent Vaccine, created by Arcturus Therapeutics Holdings, Inc., is categorized as an mRNA vaccine and functions as a preventive measure. It targets the SARS-CoV-2 S protein, making it well-suited for combating infectious and respiratory diseases, especially COVID-19 and Severe Acute Respiratory Syndrome.
The approval of this Self-Amplifying mRNA Ancestral-strain Monovalent Vaccine marks a significant advancement in addressing the COVID-19 pandemic and associated respiratory illnesses. Consequently, it is projected to greatly influence public health efforts and the global initiative to manage and halt the spread of infectious diseases.
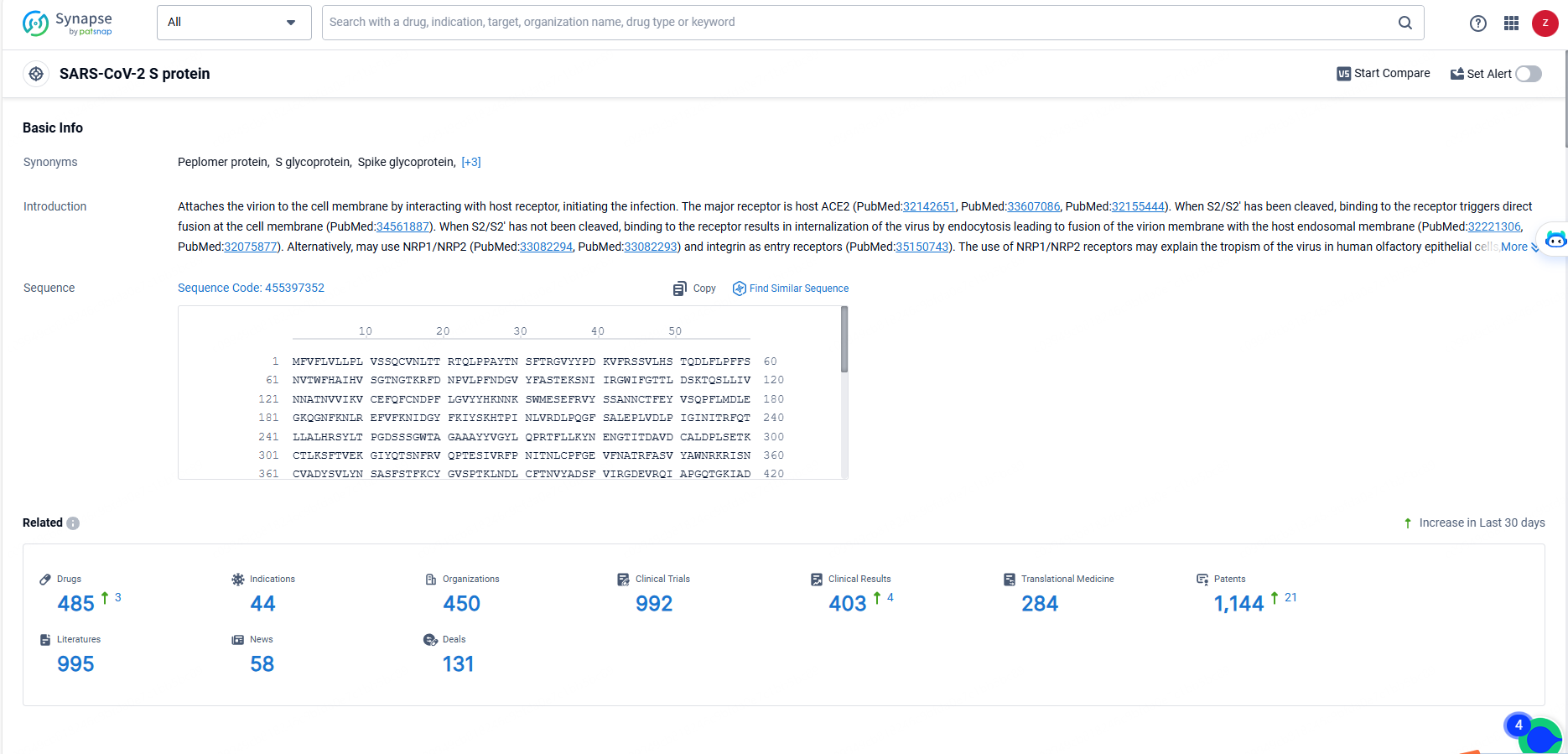
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 23, 2024, there are 485 investigational drugs for the SARS-CoV-2 S protein targets, including 44 indications, 450 R&D institutions involved, with related clinical trials reaching 992, and as many as 1144 patents.
The approval of the Self-Amplifying mRNA Ancestral-strain Monovalent Vaccine signifies a significant achievement in the pharmaceutical industry, with the potential to make a meaningful difference in the lives of individuals affected by COVID-19 and other respiratory diseases.