Neurogene Updates on NGN-401 Clinical Trial for Rett Syndrome Gene Therapy
Neurogene Inc. (Nasdaq: NGNE), a clinical-stage organization established to deliver transformative genetic therapies to individuals and families impacted by rare neurological disorders, has provided an update regarding its ongoing Phase 1/2 open-label clinical study assessing the NGN-401 gene therapy aimed at treating Rett syndrome.
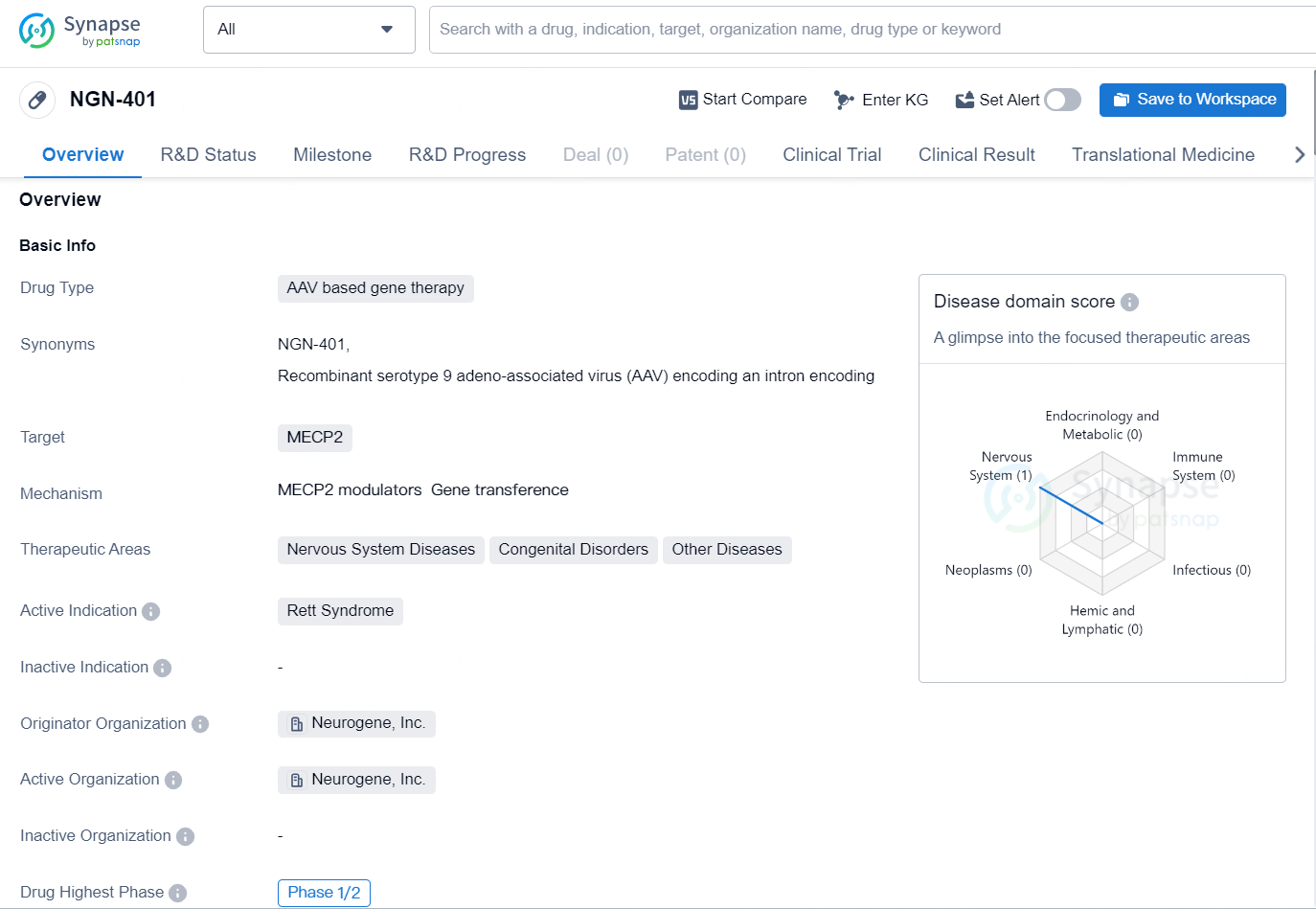
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
As previously communicated, on November 11, 2024, Neurogene became aware of a serious treatment-related adverse event (SAE) affecting a participant in a clinical trial who had received NGN-401 at a dosage of 3E15 vg (high-dose cohort). This individual, who was administered the drug on November 5, showed signs of a systemic hyperinflammatory syndrome, which is a rare and potentially fatal immune response that has been noted with high-dose AAV systemic exposure. Hyperinflammatory syndromes involve abnormal cytokine release and can manifest as hemophagocytic lymphohistiocytosis (HLH) and multisystem inflammatory syndrome. The participant’s condition is critical, and the situation is still developing.
“We are truly heartbroken for the family. Although no words can adequately comfort them, we invite the Rett syndrome community to share their compassionate thoughts with the family, friends, and the dedicated healthcare professionals attending to her,” stated Rachel McMinn, Ph.D., Founder and CEO of Neurogene. “Ensuring the safety of our clinical trial participants remains our highest priority as we strive to develop treatments for this devastating condition.”
In the interest of complete transparency with the U.S. Food and Drug Administration (FDA), Neurogene took proactive steps to engage with the FDA through the START program following the notification of the SAE. The FDA reviewed the safety data for NGN-401 and permitted Neurogene to advance with the Phase 1/2 trial using the 1E15 vg dosage (low-dose cohort). Neurogene has suspended further administration of the 3E15 vg dosage (high-dose cohorts) following the initial report of the SAE and will not recruit any additional participants at this dosage level.
To date, there have been no other treatment-related SAEs reported in the clinical trial, including among the five participants who received the 1E15 vg dose (low-dose cohort) and the first two participants who were administered the 3E15 vg dose (high-dose cohort) of NGN-401. All treatment-related adverse events (AEs) in the 1E15 vg cohort have been classified as Grade 1 (mild). Most of these treatment-related AEs are recognized potential risks associated with AAV, have been treated with steroids, and have either resolved or are in the process of resolving. There have been no indications of toxicity related to MeCP2 overexpression. Moreover, there have been no AEs associated with intracerebroventricular (ICV) procedures.
Neurogene no longer expects to complete enrollment in the 1E15 vg cohort (low-dose cohort) of NGN-401 in the fourth quarter of 2024, as the Company revises the protocol to account for the discontinuation of the 3E15 vg dosage.
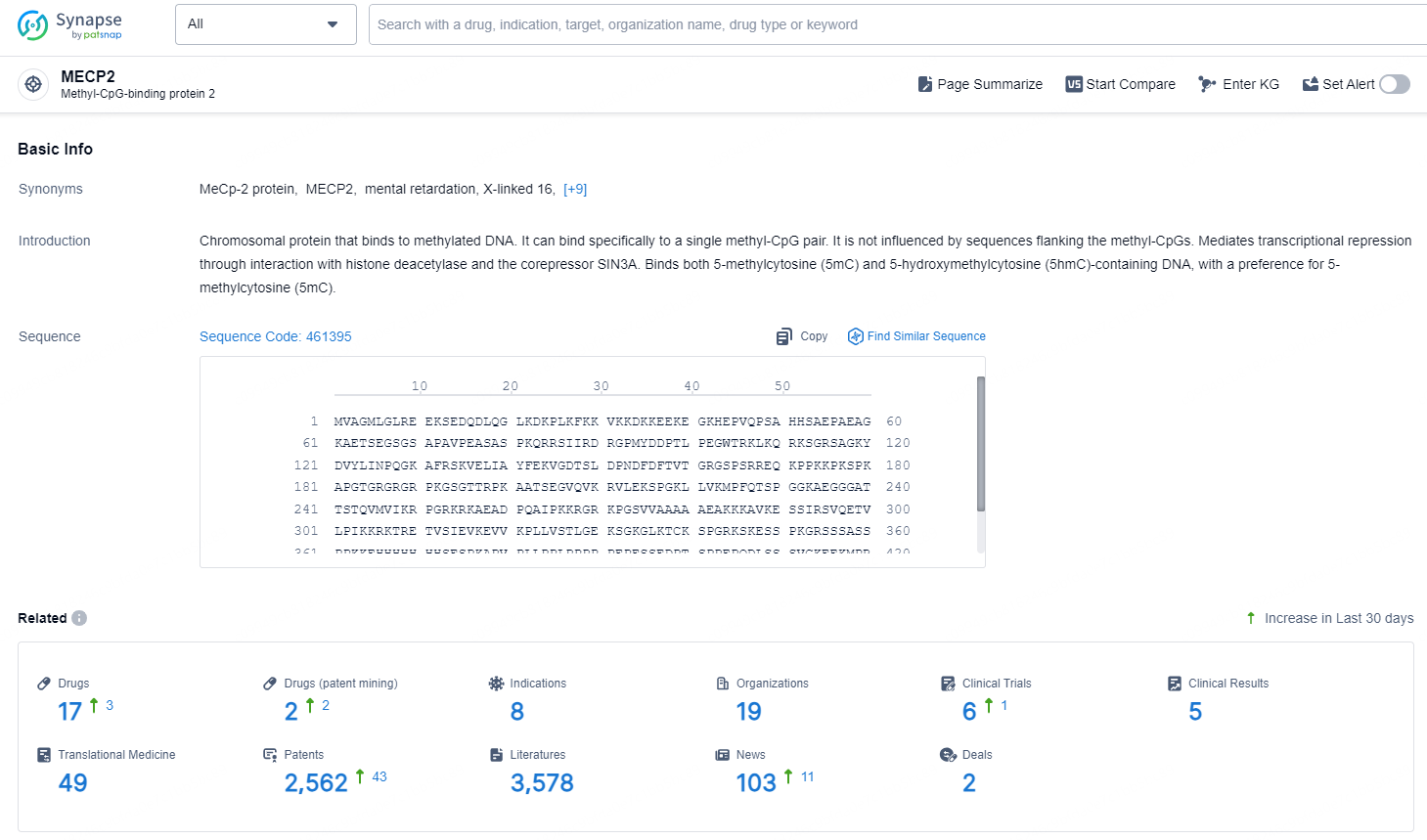
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of November 20, 2024, there are 17 investigational drugs for the MECP2 target, including 8 indications, 19 R&D institutions involved, with related clinical trials reaching 6, and as many as 2562 patents.
NGN-401 is an AAV based gene therapy drug developed by Neurogene, Inc. The drug targets MECP2 and is primarily focused on treating Rett Syndrome, a rare neurological disorder that predominantly affects females. Rett Syndrome is characterized by a loss of acquired skills and motor function, as well as the development of repetitive hand movements.