Novartis continues to increase its investments in the "cyclic peptide + radiopharmaceuticals" therapeutic field
On May 2nd, Novartis announced the official signing of an agreement to acquire Mariana Oncology located in Watertown, Massachusetts, USA. According to the terms of the agreement, Novartis will initially pay $1 billion and, upon achieving predefined milestone objectives, will pay an additional $750 million.
Mariana is a preclinical-stage biotechnology company focused on developing novel radioligand therapies for cancers with high unmet medical needs. This acquisition will not only enrich Novartis's research and development pipeline in Radioligand Therapy (RLT) but will also enhance the company’s research foundation and clinical supply capabilities in oncology and RLT platform innovations, showcasing its strategic focus in the field of cancer treatment.
Radioligand therapy is a form of precision medicine that involves coupling tumor-targeting molecules (ligands) with therapeutic radioactive isotopes to precisely target specific tumor surface receptors. Once bound to the target cells, the radioactive isotopes release radiation that causes DNA damage, inhibits cell proliferation, and may induce cell death, thereby delivering radiation directly to the tumor while minimizing damage to surrounding healthy tissue.
It is reported that Mariana Oncology has joined Novartis with a series of Radioligand Therapy (RLT) projects that span multiple solid tumor indications, such as breast cancer, prostate cancer, lung cancer, etc., including the candidate drug MC-339 for small cell lung cancer research. MC-339 is a radio-ligand therapy based on the actinide elements.
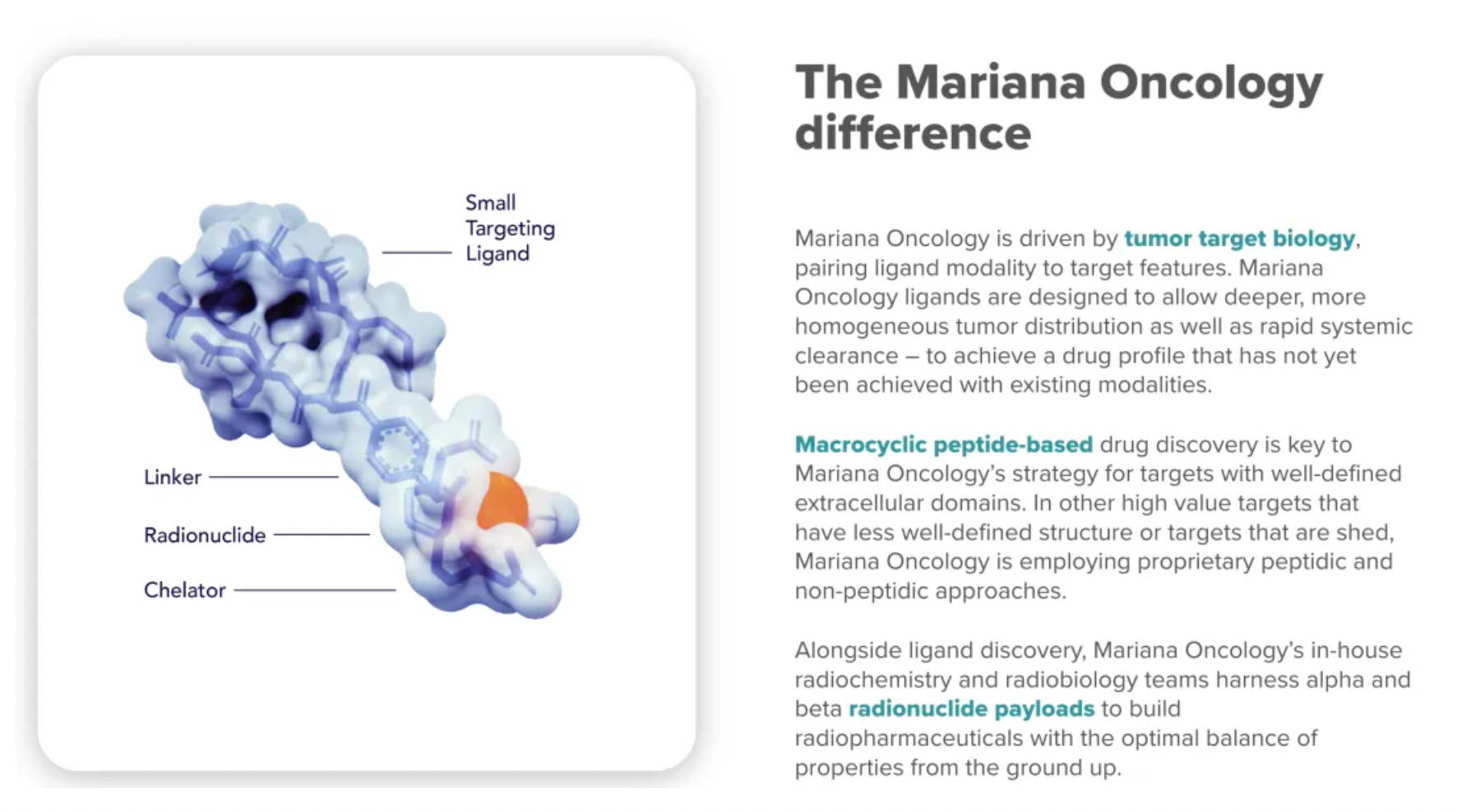
Mariana Oncology, with its strategy centered on driving the biology of tumor targets, precisely matches ligand modalities with target characteristics. For targets with clear extracellular domains, drug discovery based on macrocyclic peptides is key to Mariana's strategy. For high-value targets with poorly defined structures or prone to shedding, the company employs unique peptide and non-peptide methods for research.

Beyond ligand discovery, Mariana’s in-house radiochemistry and radiobiology team utilizes alpha and beta radioactive nuclides as effective payloads to build radiopharmaceuticals from scratch that balance optimal properties. The standards for ligand design include maximum efficacy, high selectivity, stability, physicochemical multiparameter optimization, and the avoidance of drug efflux/uptake transporters. This is complemented by linker and chelation chemistry techniques to carry radioactive active components. With a solid R&D background and flexible operations in peptide and non-peptide small molecule drug discovery, Mariana Oncology tactfully balances these elements.
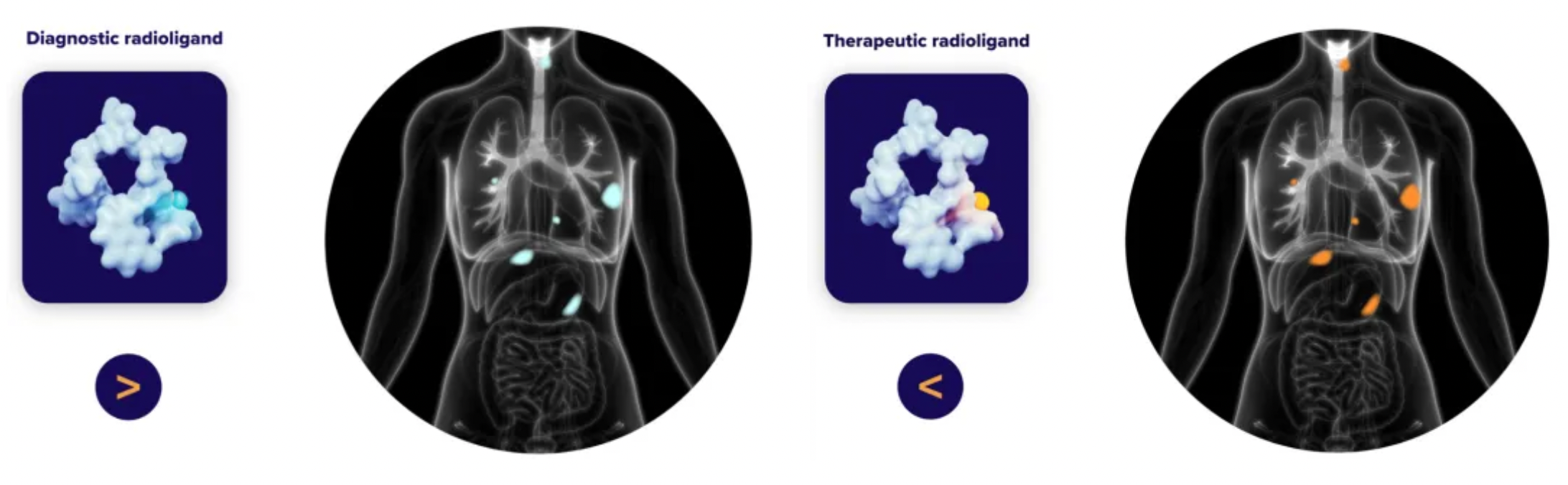
Radiopharmaceuticals enable personalized treatment strategies to be integrated into clinical development and subsequent commercialization processes. The same ligand can be loaded with therapeutic radioactive nuclides (therapeutic type) or diagnostic/imaging radioactive nuclides (diagnostic type). By adopting a 'theranostic' strategy, we confirm target binding in the clinical development phase, select appropriate patient groups, and optimize dosing, thus maximizing therapeutic effects. This strategy, combining diagnostic and therapeutic dual functions, not only helps in precise disease diagnosis but also customizes treatment plans based on individual differences, representing an important aspect of precision medicine.
Novartis Expands Its Nuclear Medicine Collaboration with PeptiDream
On April 30, the Japanese biopharmaceutical company PeptiDream announced that it has further expanded its collaboration with Novartis in the field of peptide discovery. Under this multi-project collaboration agreement, PeptiDream will use its Peptide Discovery Platform System (PDPS) technology to identify and optimize macrocyclic peptides targeting specific molecules selected by Novartis. The peptides developed will be conjugated with radioactive isotopes to form Radioligand Therapies (RLTs) or other applications for therapeutic and diagnostic purposes.
PeptiDream will receive an upfront payment of approximately $180 million from Novartis, along with the opportunity to earn milestone payments of up to $2.7 billion upon achieving various research, regulatory, and commercial goals. Furthermore, Novartis will also pay royalties to PeptiDream based on the tiered net sales of the products. This collaboration deepens the partnership between the two companies in the development of peptide-based drugs, aimed at fostering the creation of more innovative therapies, particularly in the field of radioligand therapies, to meet unmet medical needs.
PeptiDream is a biotechnology company headquartered in Japan, leading the transition to a new class of innovative drugs in the macrocyclic peptide sector, aiming to address unmet medical needs and improve the quality of life for patients worldwide.
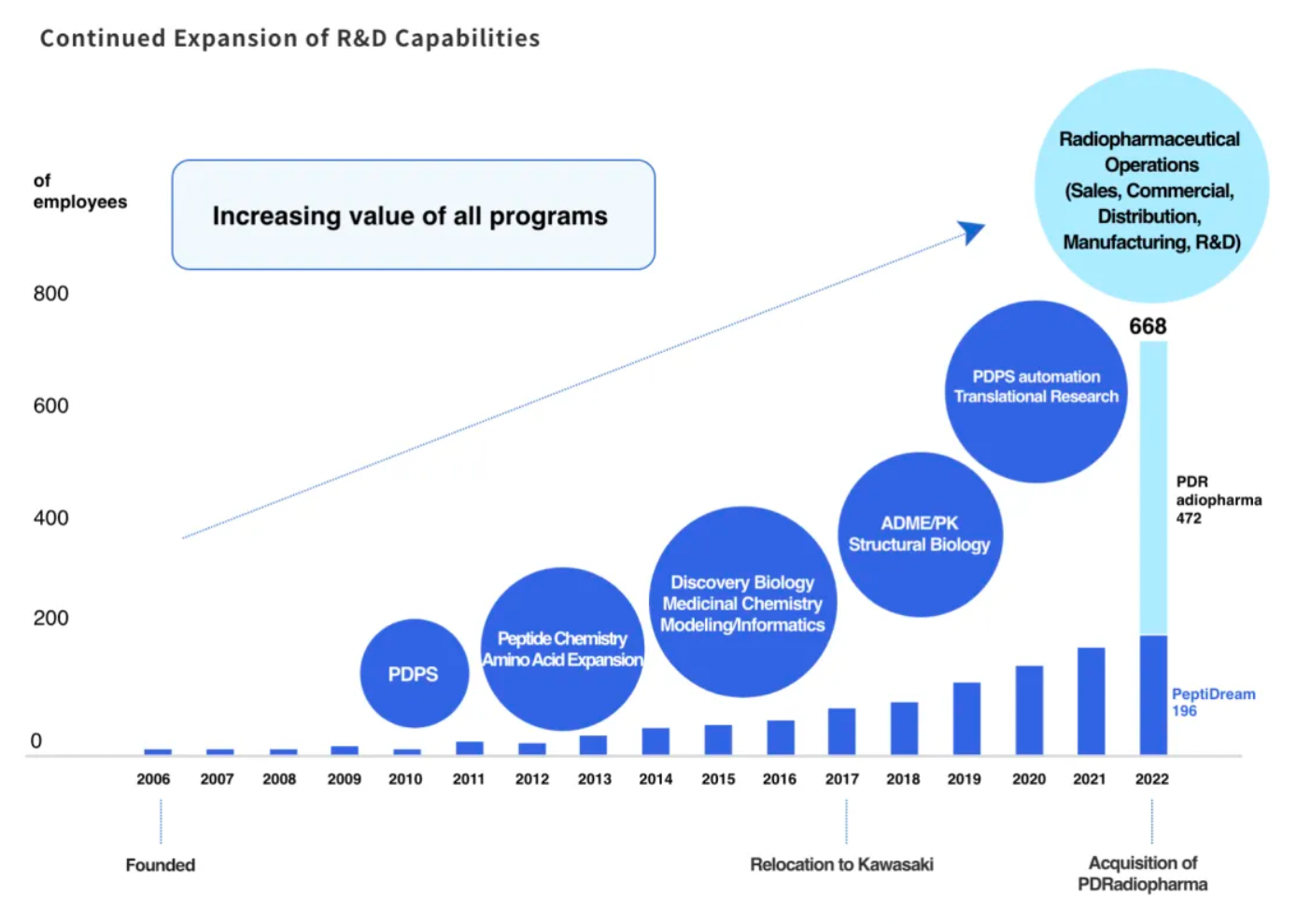
Since its inception in 2006, PeptiDream has utilized its proprietary Peptide Discovery Platform System (PDPS) technology, an advanced and highly flexible discovery platform capable of efficiently generating highly diverse (trillions of) non-standard peptide libraries for identifying highly potent and highly selective macrocyclic peptide candidate molecules.
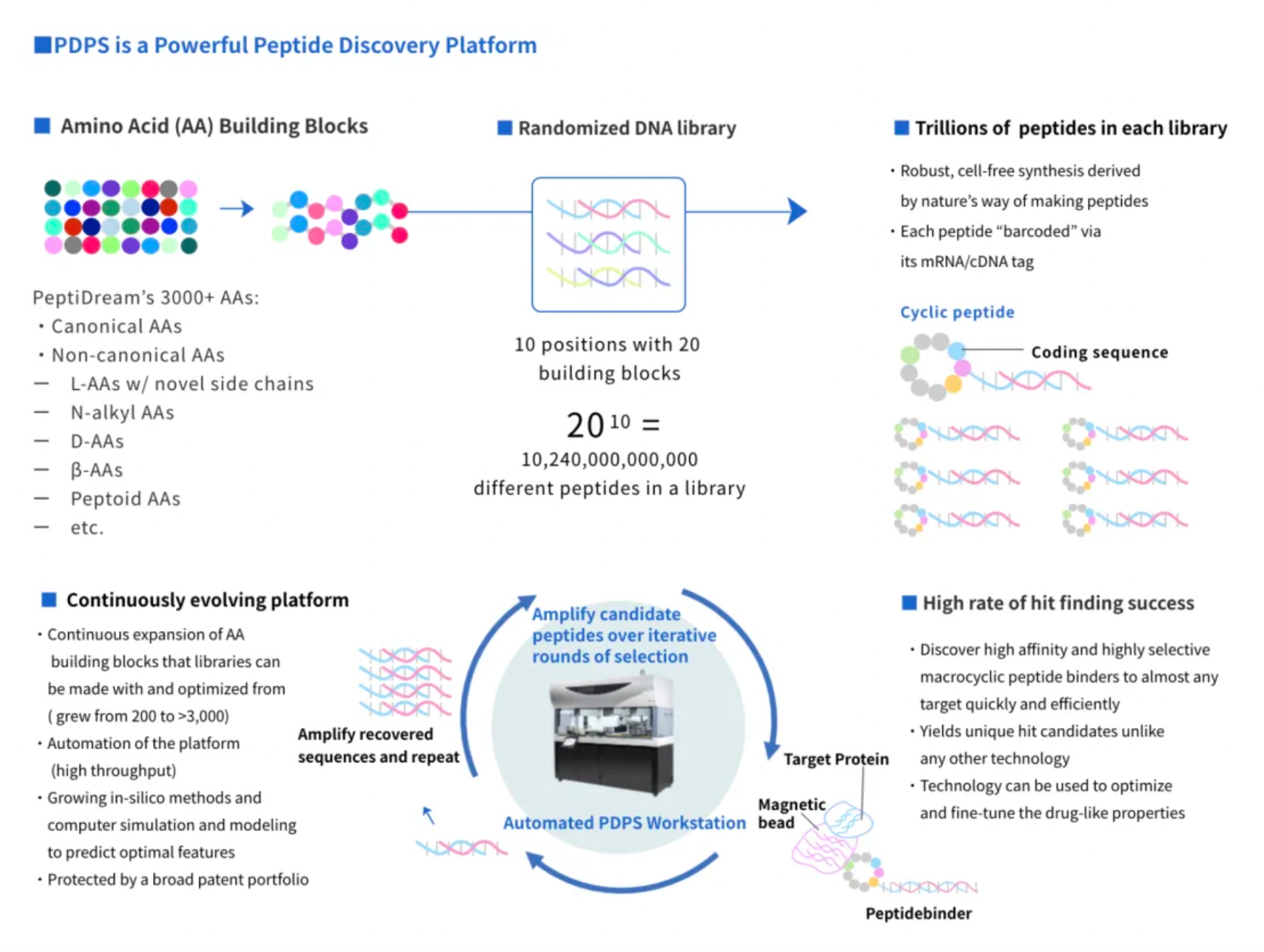
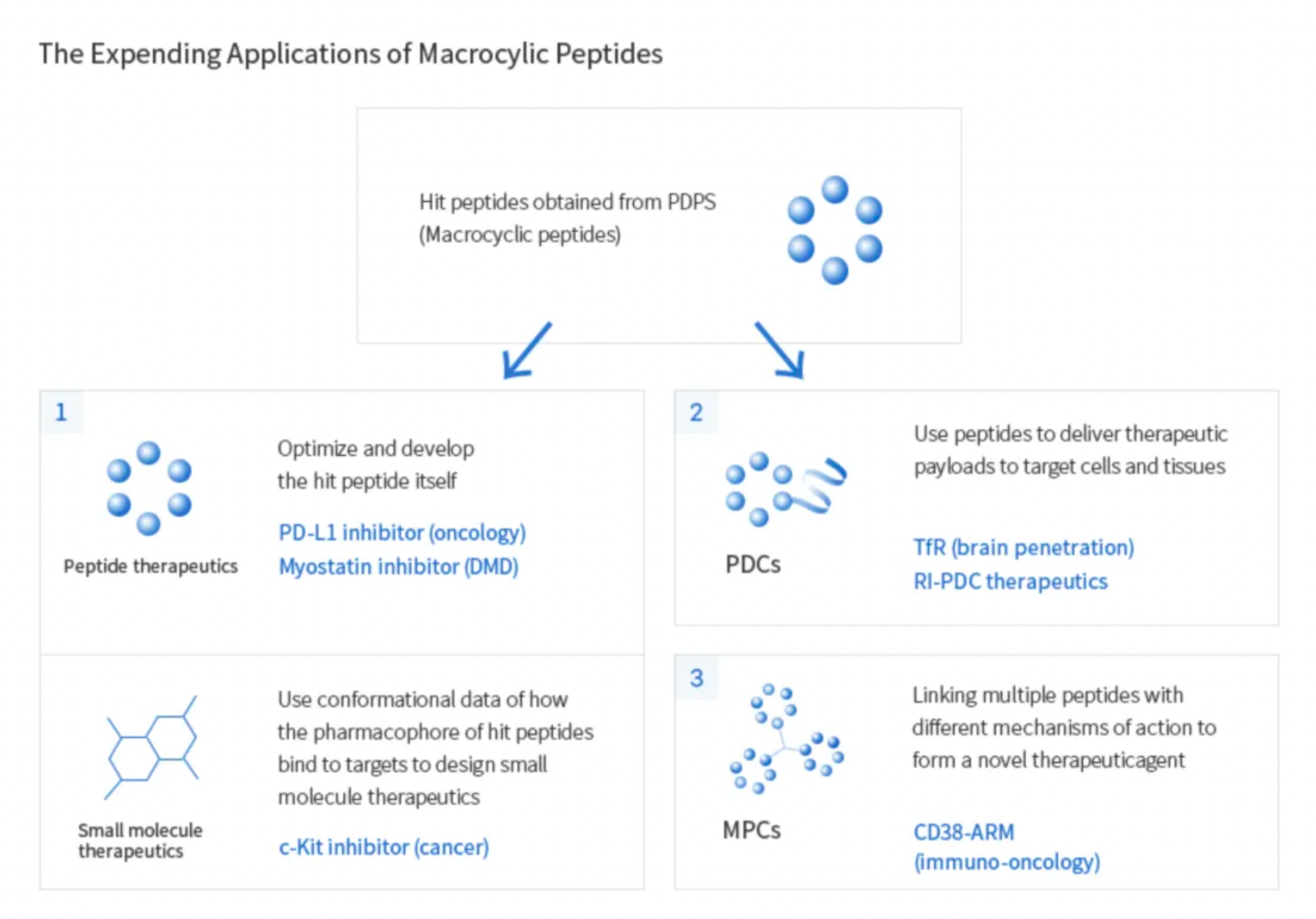
These candidate molecules can subsequently be developed into therapeutic and diagnostic products based on peptides, small molecules, peptide-drug conjugates (PDCs), and multifunctional peptide conjugates (MPCs).
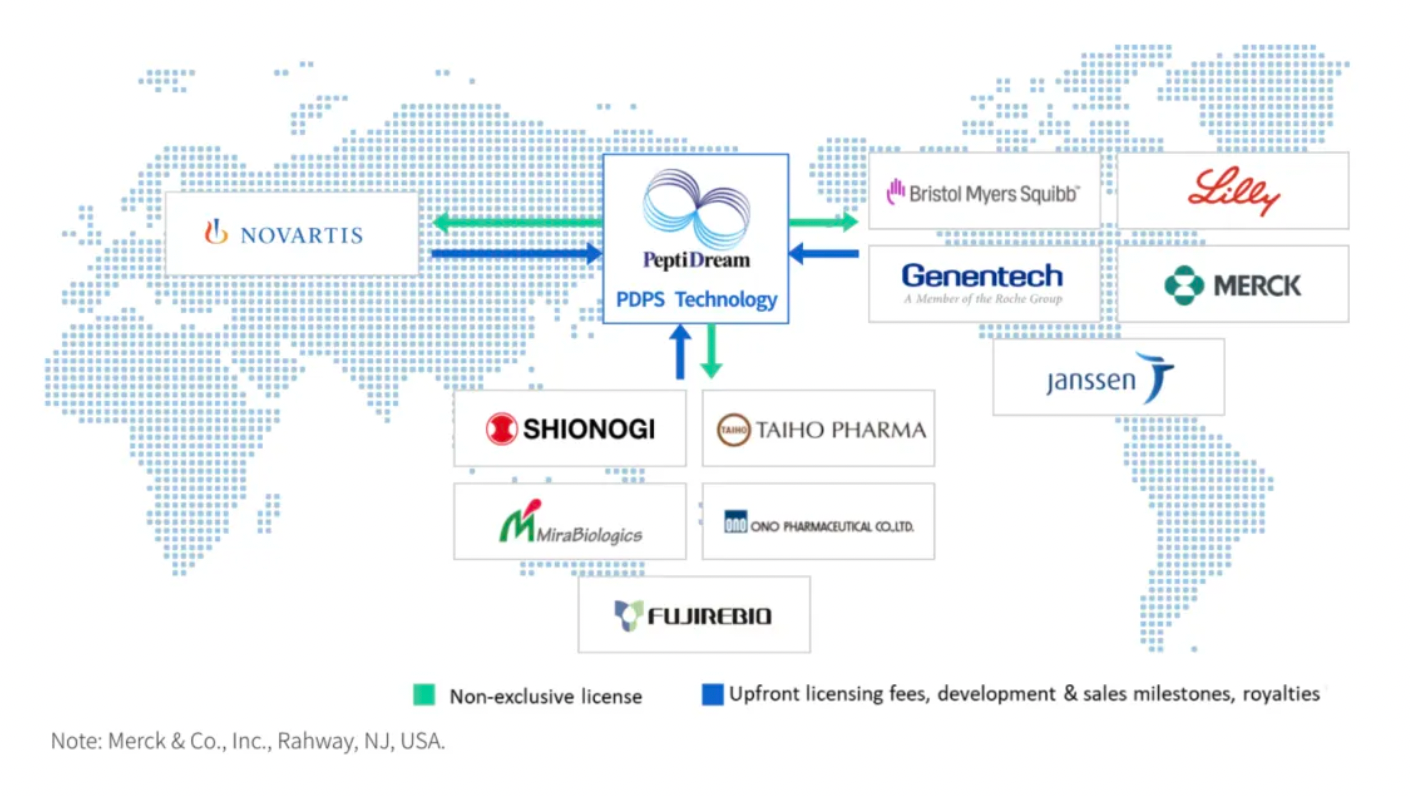
PeptiDream has established an extensive global R&D partnership network, facilitating the development and commercialization of a diverse and varied pipeline of investigational therapeutic drugs. Furthermore, through its wholly owned subsidiary PDRadiopharma, PeptiDream markets and sells several radiopharmaceuticals and radiodiagnostic products in the Japanese market.
Novartis's Blueprint for Nuclear Medicine
Novartis is attempting to consolidate its leadership in the field of radioligand therapies (RLTs).

Currently, this highly sought-after track has become a battleground for MNCs, with various companies making major deals. For example, Bristol-Myers Squibb (BMS) spent $4.1 billion to acquire RayzeBio, Eli Lilly invested $1.4 billion to purchase POINT Biopharma, and AstraZeneca acquired Fusion Pharmaceuticals for $2 billion.
As a global leader in nuclear medicine, Novartis has several drugs targeting SSTR receptors that have been approved and are on the market, including 5 approved projects and 2 Phase II clinical projects.
In October 2017, Novartis acquired the French innovative drug company Advanced Accelerator Applications for $3.9 billion to establish and expand its radioligand therapy platform. In 2018, Novartis made another acquisition worth $2.1 billion of the biopharmaceutical company Endocyte, gaining several more radiopharmaceutical candidates.
Through these acquisitions, Novartis established and expanded its radioligand therapy platform, and now has several market products such as Lutathera (177Lu-DOTATATE) and 68Ga-Edotreotide, as well as numerous products in development, including 177Lu-PSMA-617, 177Lu-PSMA-R2, 177Lu-NeoB, 177Lu-FF58, and 255Ac-PSMA-617.