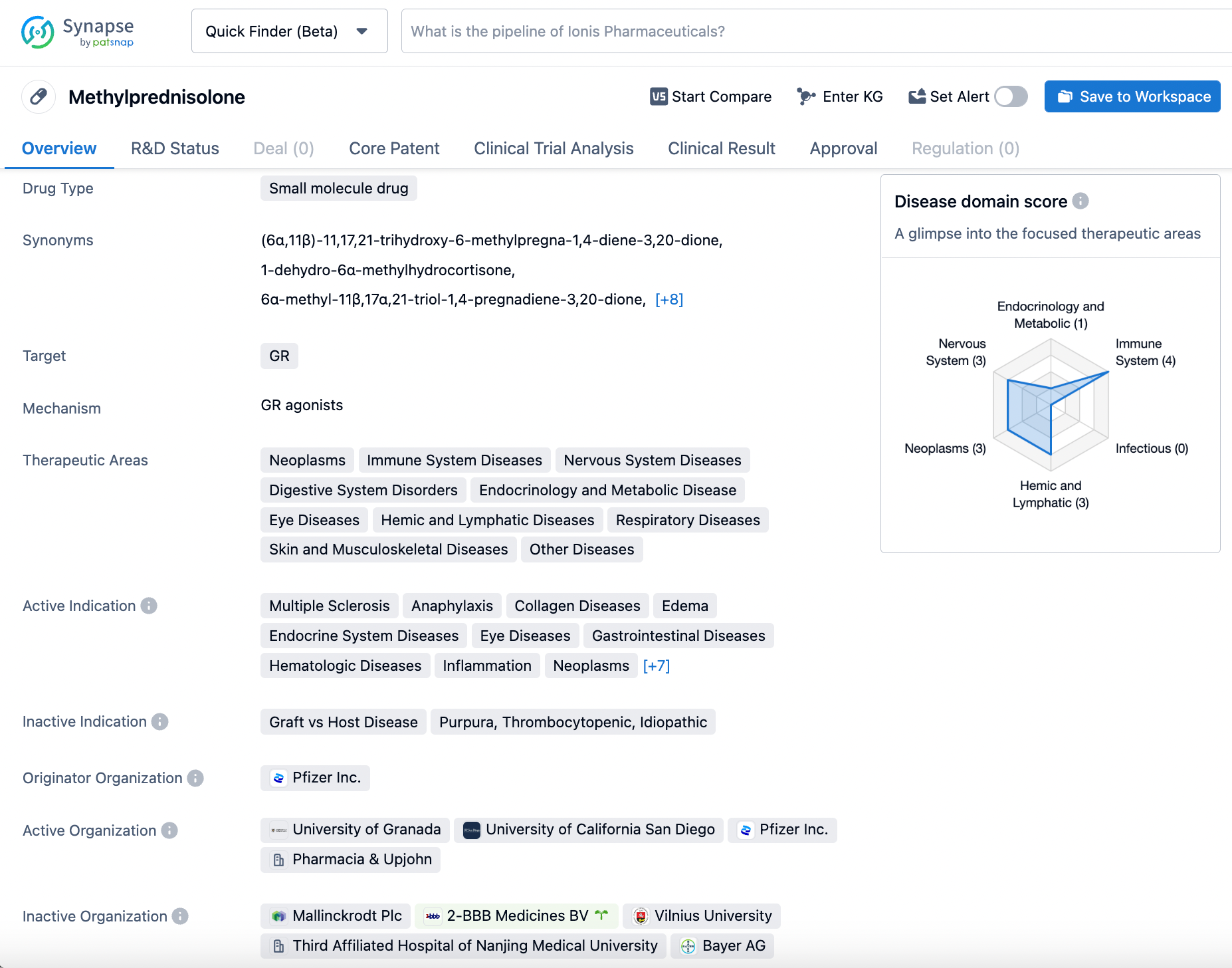
Optimize Your Synapse Experience: A Step-by-Step Guide to Searching Methylprednisolone
Methylprednisolone is a perplexing small molecule drug that targets both the NMDA receptor as a modulator and the glucocorticoid receptor as an agonist. This dual mechanism of action allows for its primary use in treating an array of conditions such as multiple sclerosis, edema, hypersensitivity, and inflammation. Methylprednisolone was first approved by the FDA on October 24th, 1957, and was initially developed by the esteemed pharmaceutical company Pharmacia & Upjohn. The drug's impact on the immune system is what makes it so potent. Methylprednisolone suppresses the immune system's response to inflammation, leading to a reduction in swelling and pain. Additionally, the drug can benefit the nervous system by decreasing nerve damage and inflammation in patients with multiple sclerosis. However, as with most medications, there is a trade-off. Methylprednisolone can also cause some unwanted side effects such as gastrointestinal bleeding and an increased risk of infection. Therefore, it is crucial to take this drug only under the guidance of a healthcare provider, and patients should be continuously monitored for any adverse effects. Click on the image below to begin the exploration journey of Methylprednisolone through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!