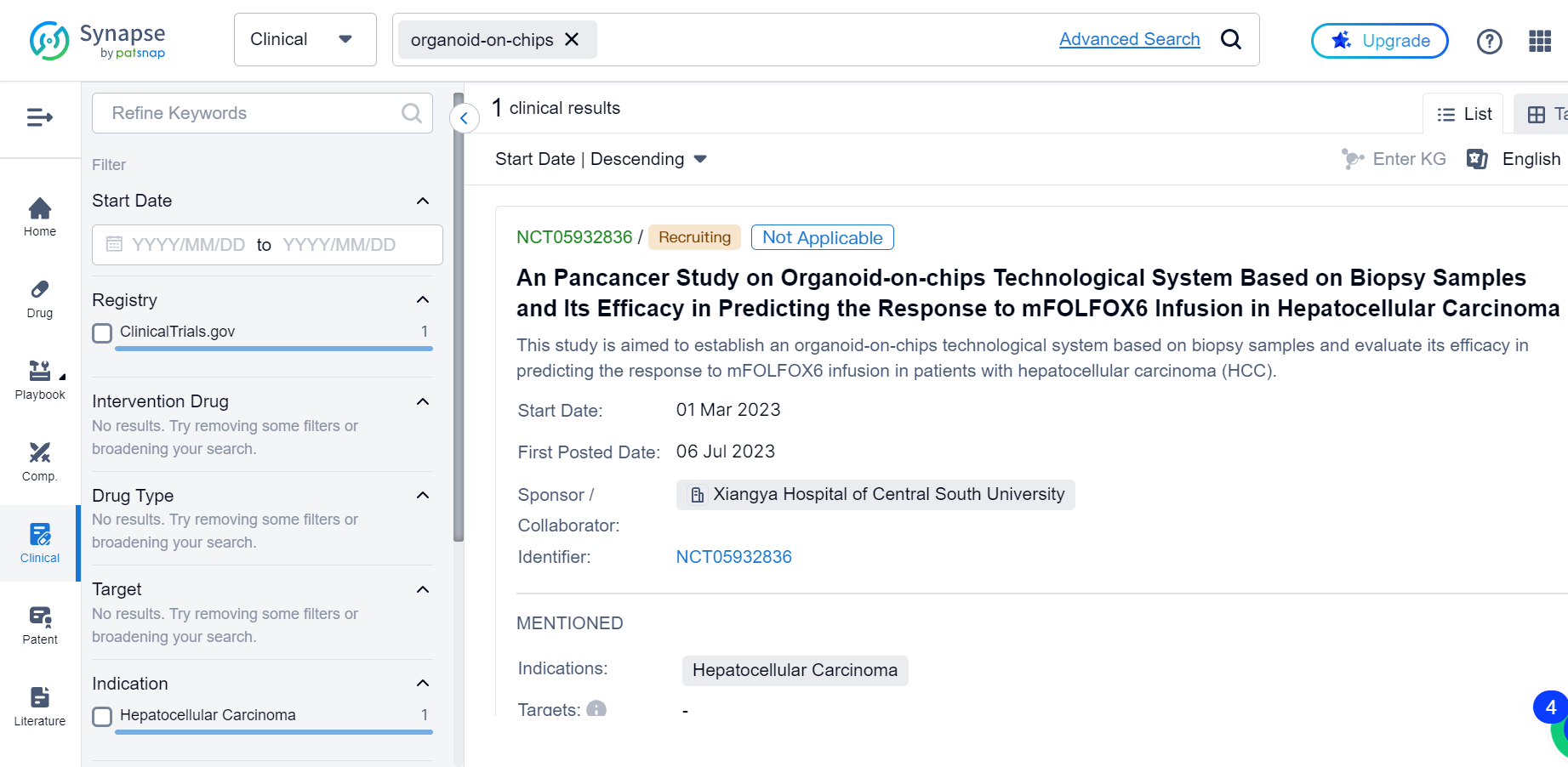
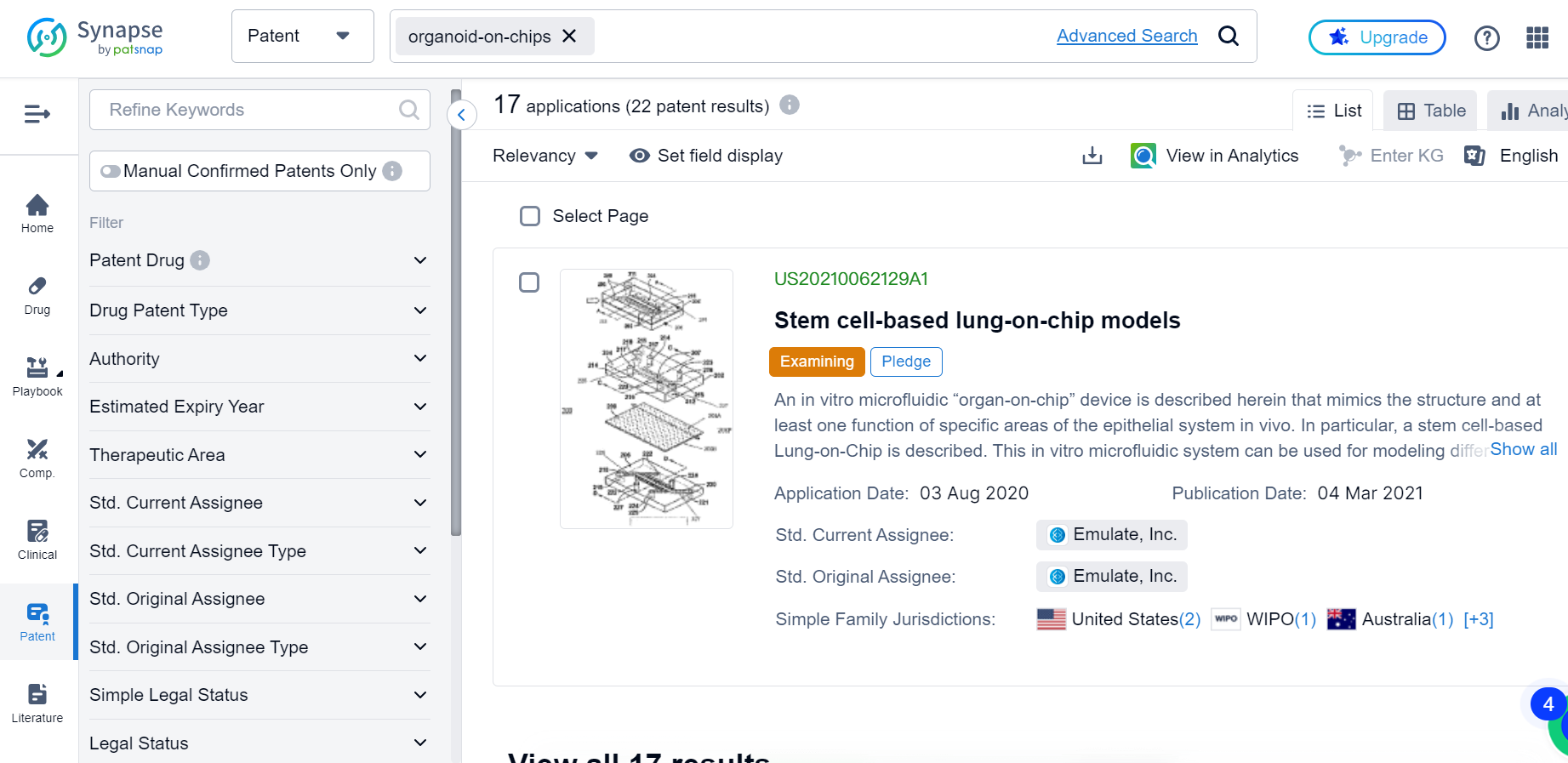
Organoid-On-Chips: A Potential Game Changer for Clinical Studies
China's first drug to be approved for clinical trials using cardiac organoid-on-chips data has been granted permission in late May 2023. According to the website of the National Medical Products Administration, Hengrui Medicine's HRS-1893 tablets have been approved for clinical trials. HRS-1893 works through a special mechanism that inhibits myocardial excessive contraction and is intended for the treatment of hypertrophic cardiomyopathy and heart failure caused by myocardial hypertrophy. This marks the first new drug in China to be approved for IND using cardiac organoid-on-chip data.
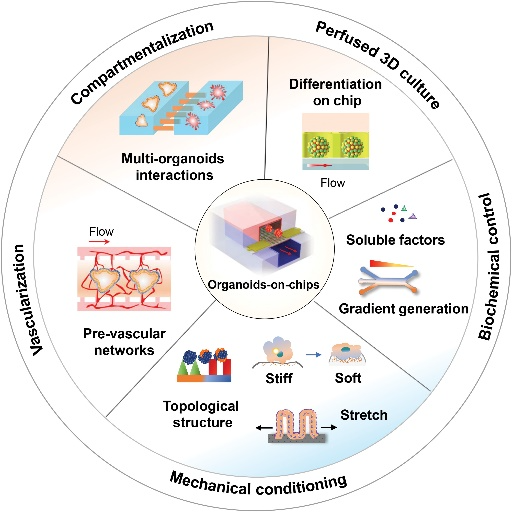
A promising alternative
The in vitro screening work of this study was based on organoid-on-chip (OOCs) technology to evaluate the effects of drugs on the contraction amplitude and calcium transient peak of cardiac organoid chips. A total of nine batches of hundreds of compounds were screened. "This study efficiently and accurately completed the screening of compound in vitro activity and drug selectivity, and found candidate molecules for later in vivo efficacy experiments," said Feng Jun, Vice President of Hengrui R&D Center in Shanghai.
This has certainly opened the door for more to follow. A search in the Synapse database shows that there’s already another drug candidate currently in clinical trial using OOCs as of July 6.
Despite still being in its early stages, the application of OOCs in clinical trials has gained significant momentum recently, with patents abound: there are in total 22 patents documented in Synapse so far.
OOCs technology involves culturing organoids on a microfluidic chip that mimics the microenvironment of the organ. The chip provides a controlled cellular microenvironment that allows the organoids to grow and develop in a physiologically relevant manner.
Application in preclinical study
An earlier study has reported the use of OOCs in predicting the efficacy of systemic treatment of colorectal cancer. Due to the heterogeneity of cancer, researchers have been working to develop personalized therapies for cancer treatment. Traditional cancer treatments have not been effective in treating cancer heterogeneity, which is why personalized therapy has emerged as a significant research focus in recent years.
Cancer-related therapeutic models, including cell lines, patient-derived xenografts, and organoids, are being developed. Organoids, three-dimensional in vitro models that replicate the cellular and molecular composition of the original tumor, have demonstrated significant advantages for developing personalized anticancer therapies, including preclinical drug screening and the prediction of patient treatment response.
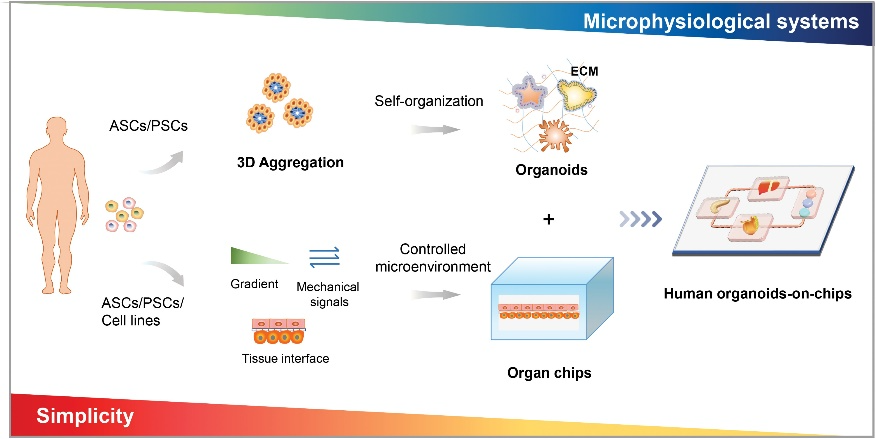
The impact of the microenvironment on cancer treatment cannot be underestimated, and OOCs can simulate the microenvironment by combining two or more cell types, allows organoids to interact with other technologies.
In the study, researchers examined the efficacy of two preclinical models, the adult stem cell-derived organoid system and OOCs technology, in predicting systemic treatment of colorectal cancer. They focus on the consistency of in vitro and in vivo drug responses based on these two models and the feasibility of adjusting treatment strategies in clinical patients based on this predictive effect as a supplement to the clinical treatment reference index. The researchers also discuss the limitations of both techniques and how they complement each other well.
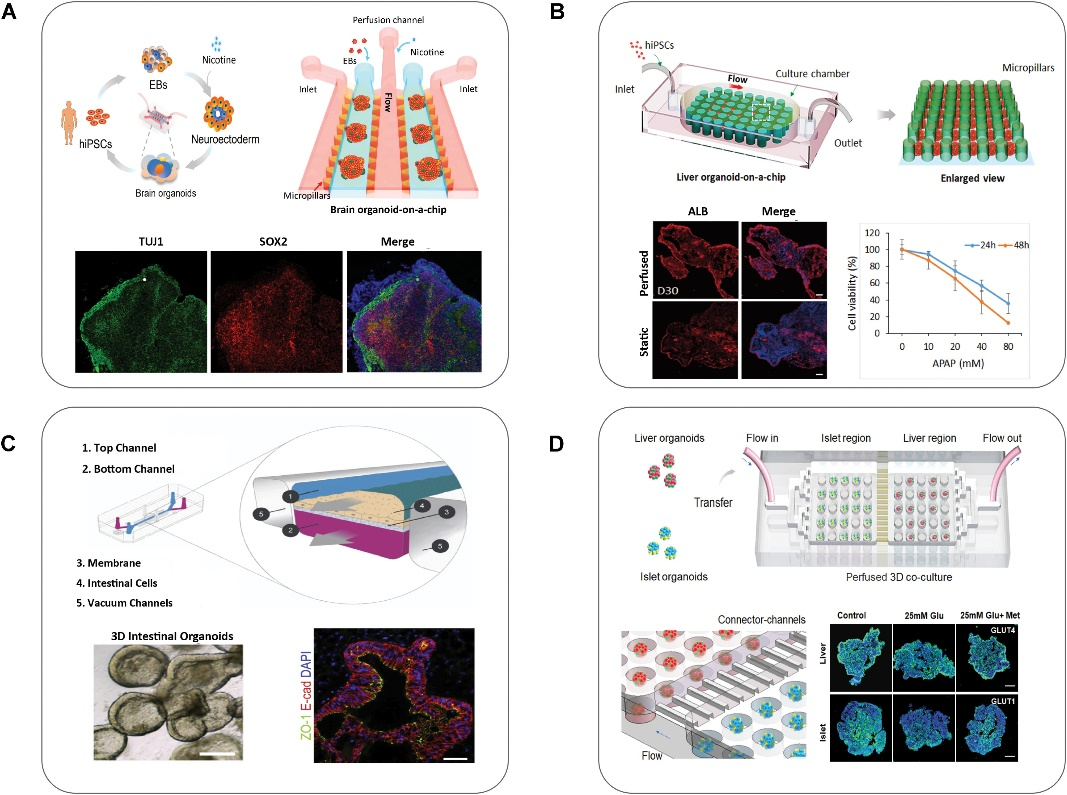
OOCs provide a preclinical system for evaluating the response to cancer therapies. Microfluidic devices combined with spheroids can mimic the tumor microenvironment for drug screening of anticancer candidates. Although organoids are a better simulation of the tumor, OOCs can assemble endotheliocyte, fibroblast, and immune cells to mimic a relatively intact tumor microenvironment. Studies have shown that OOCs can predict chemotherapy efficacy to some extent, but more studies with primary culture spheroids or cells are needed to ensure similarity to the patient's tumor. In future studies, OOCs may construct cellular components with primary culturing, microenvironment-related cells, and vasculature to evaluate drug response and predict chemotherapy efficacy.
Predicting the efficacy of colorectal cancer treatment
OOCs can also be used to screen drugs for colorectal cancer. Vascularized micro tumors (VMTs) are a representative model that can test ten kinds of clinically-approved receptor tyrosine kinase inhibitors and novel targeted drugs like galunisertib. Drug delivery and penetration have also been explored in similar colorectal cancer organ-chip systems. While the fidelity of the tumor itself is not superior to the organoid, the integrity of the microenvironment can better explore the interaction of medicine and the cell-extracellular matrix. Combining primary tumor cells can predict patient efficacy to some extent. Similar efficacy prediction studies have been conducted in other tumor chips, demonstrating the potential of exploring drug efficacy prediction and evaluation in the OOCs system, even in colorectal cancer. This highlights the use of organoids and OOCs as complementary reference tools in treating colorectal cancer from the perspective of clinical efficacy predictability.
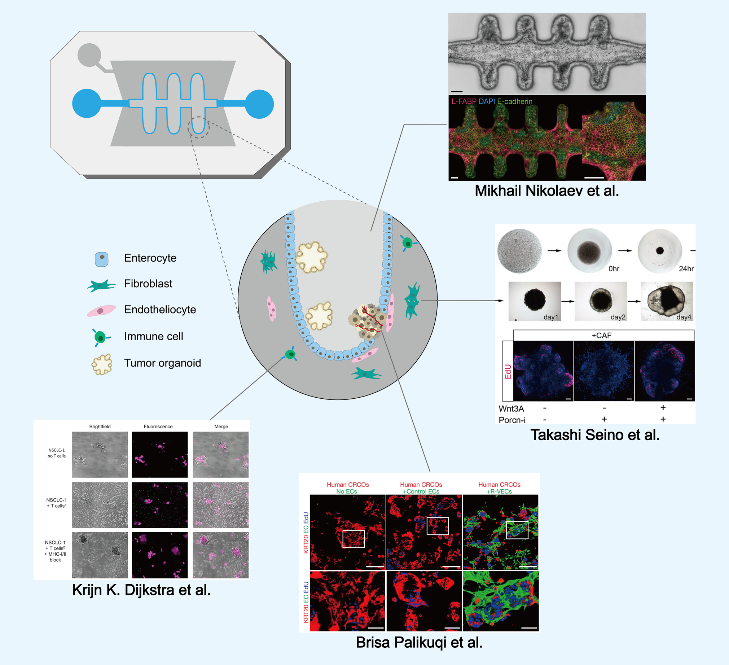
In fact, a number of clinical trials based on these models are currently underway. In terms of the treatment time window, cost, drug screening efficiency, and accuracy, these in vitro models will likely be used as a clinical reference to guide the optimization of treatment strategies for individual patients through technological advancement or technology fusion.
Similarly, other OOCs can be developed for different organs such as liver, lung, heart, kidney, etc. The technology has great potential in disease research, drug testing, and regenerative medicine. OOCs technology has the potential to revolutionize biomedical research, drug testing, and regenerative medicine. It is innovation that offers a wide range of potential applications to the field of medicine.

References
1.Zhu, J., Ji, L., Chen, Y. et al. Organoids and organs-on-chips: insights into predicting the efficacy of systemic treatment in colorectal cancer. Cell Death Discov. 9, 72 (2023).
2.Yaqing Wang , Jianhua Qin, Advances in human organoids-on-chips in biomedical research, Life Medicine, Volume 2, Issue 1, February 2023, lnad007, https://doi-org.libproxy1.nus.edu.sg/10.1093/lifemedi/lnad007