Patent Analysis of Jiangsu Hengrui's PARP1 Inhibitor HRS-1167
At the end of October, Hengrui and Merck reached a transaction cooperation totaling 1.4 billion Euros, with an initial payment of 160 million Euros. The transaction mainly includes Hengrui's independently developed PARP1 inhibitor HRS-1167 and Claudin-18.2 antibody-drug conjugate (ADC) SHR-A1904. The distribution ratio of the initial payment between small molecules and large molecules was not disclosed in the transaction. Considering the current research popularity of ADC, SHR-A1904 should have a higher proportion.
Prior to this, Hengrui's overseas cooperation projects included SHR0302, SHR1459, Apatinib, Carrelizumab, Pyrotinib, and SHR1701, among others. The small molecule with the highest upfront payment was EZH2 inhibitor SHR2254, which Hengrui licensed out in February of this year for an upfront payment of $11 million. After all, SHR2254 is already in Phase I/II clinical trials, so the upfront payment naturally wouldn't be low. SHR2254 is indicated for the treatment of conditions including B-cell lymphoma, peripheral T-cell lymphoma, prostate cancer, and solid tumors, among others. This time raising the upfront payment record to 160 million Euros is of milestone significance for Hengrui. What insights can we gain from this?
HRS-1167 is a small molecule drug, with PARP1 as its target. PARP1 is a protein associated with DNA repair and cell apoptosis, playing a crucial role in tumor development. Therefore, HRS-1167 is widely used in the field of tumor treatment.
At present, HRS-1167 is undergoing phase 1 clinical trials for solid tumors. Solid tumors are a common type of tumor, including lung cancer, breast cancer, colorectal cancer, and so on. By targeting PARP1, HRS-1167 may be able to interfere with the DNA repair mechanisms of tumor cells, leading to the death or inhibition of tumor cell growth. With further research and clinical trials, HRS-1167 is hoped to provide a new treatment option for cancer patients.
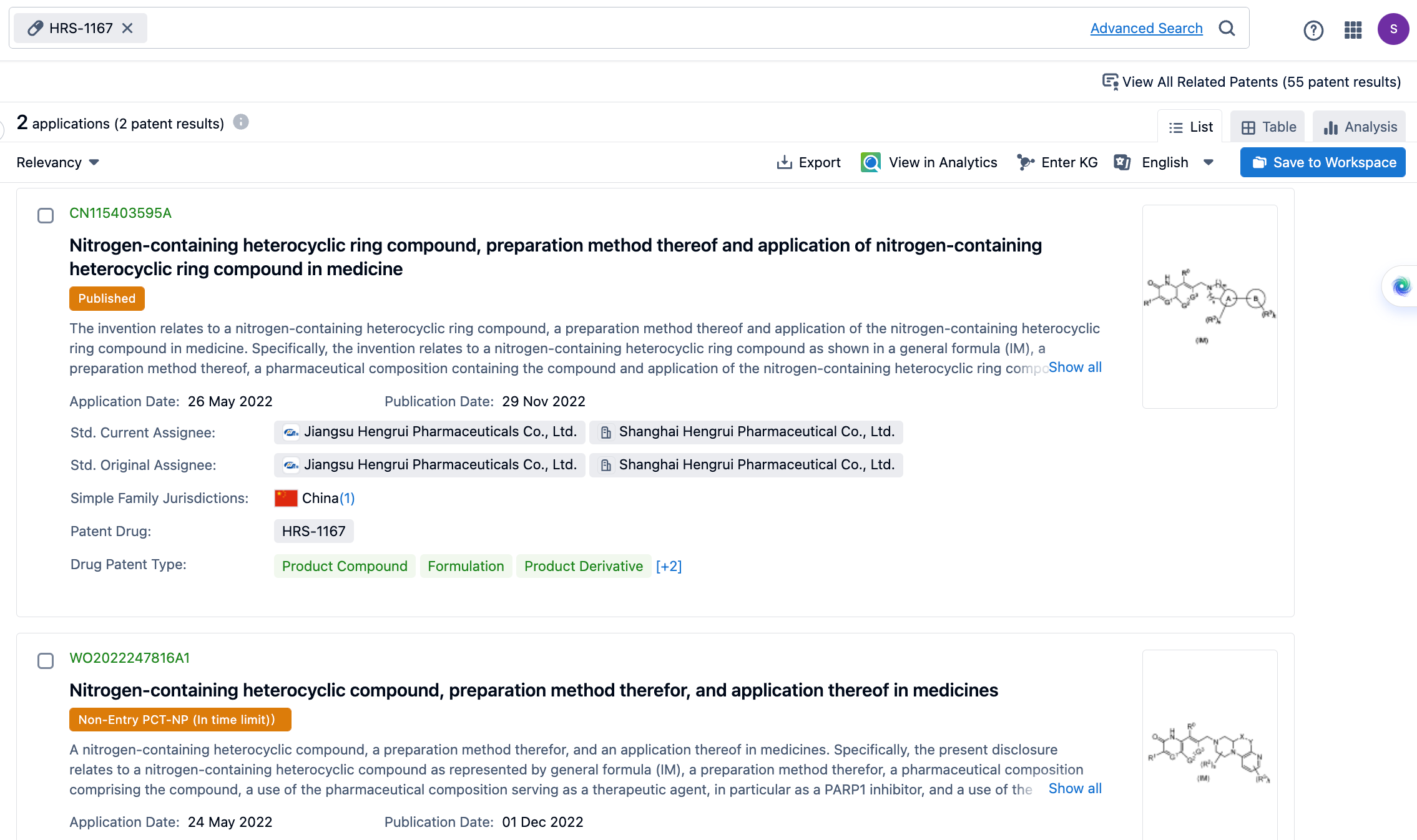
In the Synapse database, search for HRS-1167 and find two groups of patents applied for by Hengrui, which are CN115403595A and WO2022247816A1. The WO patent also has a Taiwanese homologous patent, TW202313630A. The drugs protected by these patents may all contain HRS-1167.
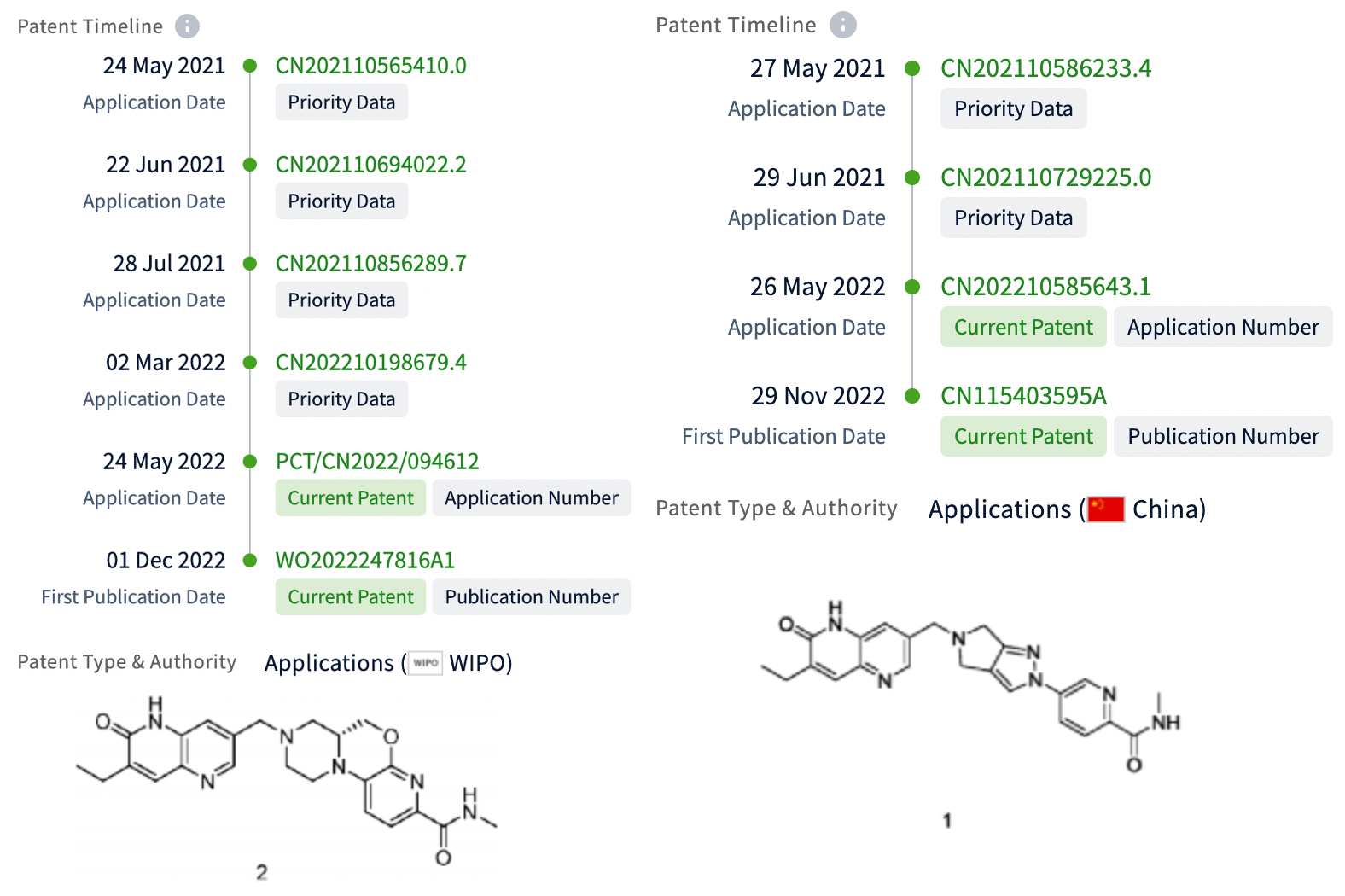
On May 24 and 27, 2021, Hengrui respectively initiated temporary applications for two patents, and obtained the corresponding patents for molecular formulas and compounds. It is clear which patent is more important. It is considered by the author that HRS-1167 is more likely to be the structure of Example 2 in WO2022247816A1.
In the WO patent, the Compound of Example 2 significantly inhibits recombinant PARP1 activity (IC50 = 11 nM), which is more selective than recombinant PARP2 (IC50= 1779 nM). In the Cell Titer-Glo assay, it showed anti-proliferative activity against human breast cancer MDA-MB-436 and colon cancer DLD-1 cells with knocked out BRCA2 (IC50 values of 1 and 2.5 nM respectively), and no significant activity against wild-type DLD-1 cancer cells (IC50>250 nM).
WO2022247816A1 includes 10 examples and corresponding isomers. CN115403595A includes 4 examples and related isomers.
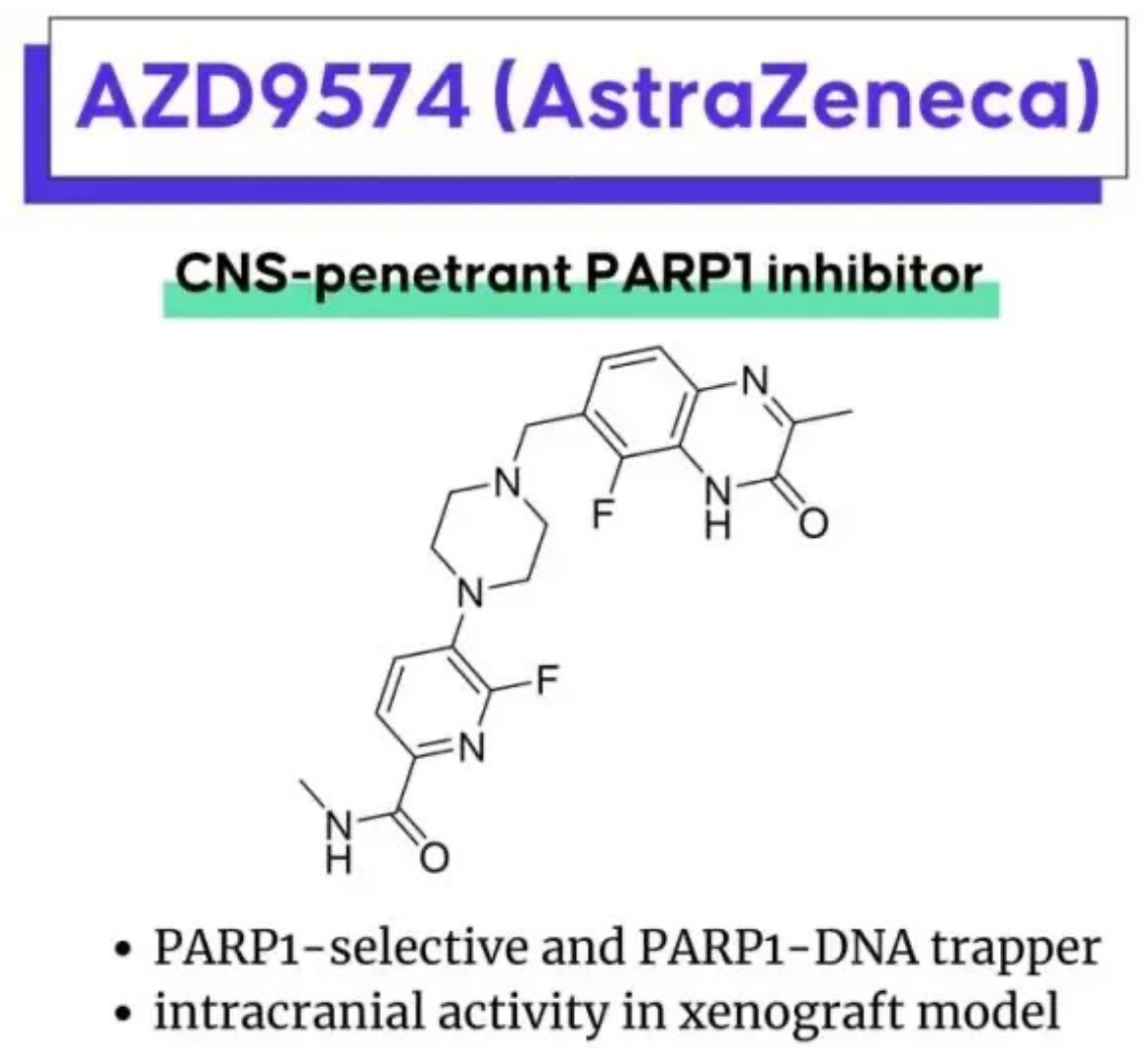
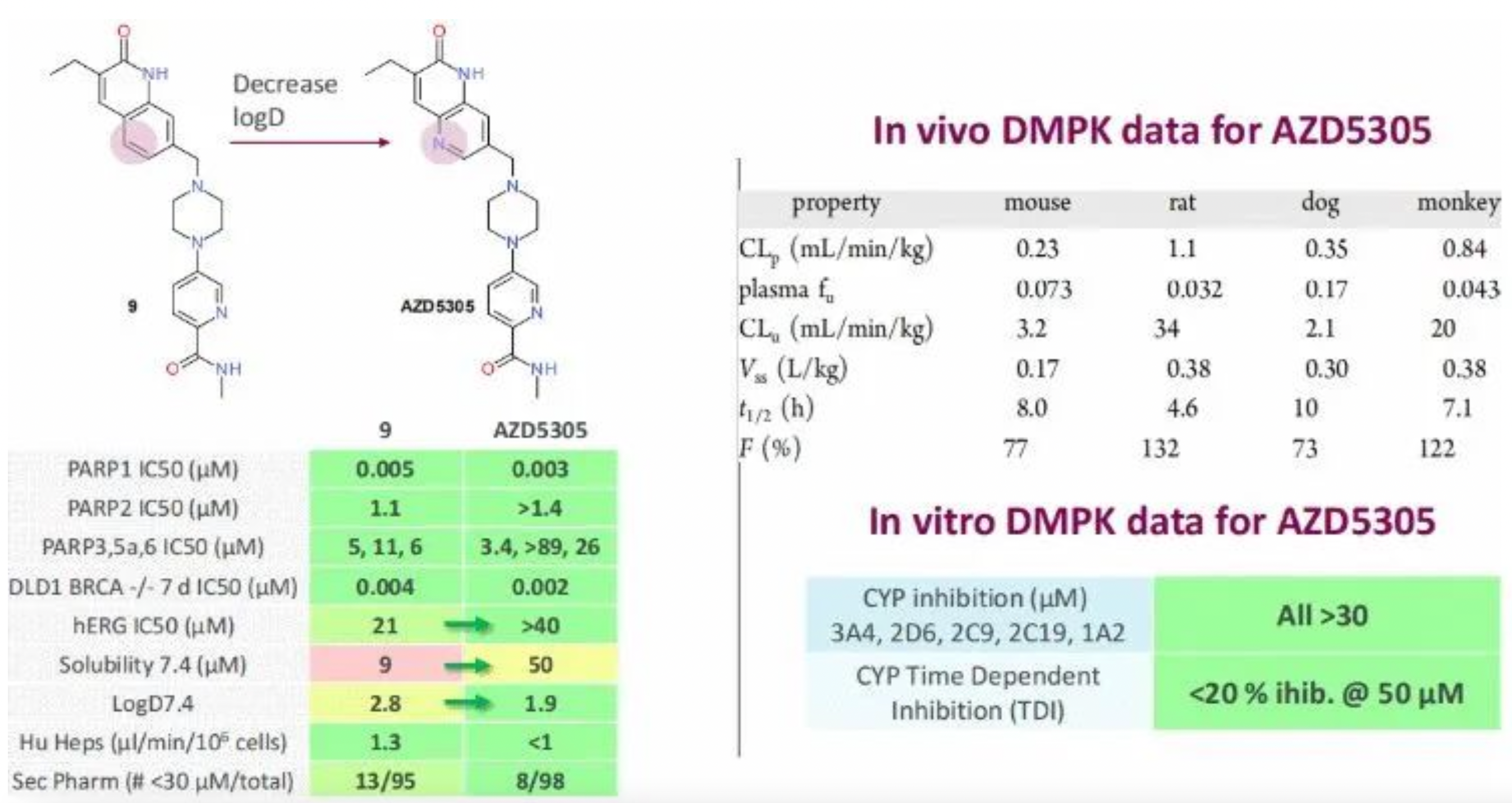
These two patents are both fast follows on AstraZeneca's PARP1 inhibitor AZD5305. Hengrui has once again maximized benefits by minimizing costs (with the synthesis of 14 examples). It is also worth admiring AstraZeneca's strong research and development prowess in PARP inhibitors. Additionally, AstraZeneca also has another brain-penetrating PARP1 selective inhibitor AZD9574, the structure of which was first revealed at the 2022 ACS Spring Meeting. It is believed that Hengrui should also have a strategic layout in the same field.
The preclinical in vivo pharmacokinetic (PK) characteristics of AZD5305 were measured in mice, rats, dogs, and cynomolgus monkeys. The compound AZD5305 has a very low plasma clearance (CLp) of 0.23, 1.1, 0.33, and 0.84 mL/min/kg respectively in mice, rats, dogs, and monkeys, while the steady-state volume of distribution (Vss) is 0.17, 0.38, 0.30, 0.38 L/kg. The plasma half-life is 8.0, 4.6, 10, and 7.1 hours respectively. The oral bioavailability of AZD5305 is very high in all species, consistent with low hepatic clearance and high absorption rates. With a relatively low unbound clearance rate, coupled with high potency, it is expected that the effective in vivo doses can be reduced.
However, the current clinical trials have not revealed the dosage of AZD5305. The clinical registration information updated in July 2023 only includes two items: NCT05938270 and NCT05797168. Among them, NCT05797168 is a phase I/IIa study of AZD5335 as a monotherapy and in combination with anti-cancer drugs for the treatment of solid tumor patients. AZD5335 is AstraZeneca's FRα ADC drug, with AZD5305 being used as a combination drug. NCT05938270 also involves combination use with darolutamide.
Moreover, the patent WO2023089527 includes an embodiment of the anti-tumor effect of AZD5305 combined with the TROP-2 ADC drug DS-1062. Patent WO2022074617 introduces the combination use of AZD5305 and DS8201. In AstraZeneca's public pipeline, AZD5305 does not seem to be given enough attention.
Based on the previous analysis, the likely structure of HRS-1167 is the molecular structure presented in Embodiment 2 of WO2022247816A1. The difference between this molecule and AZD5305 is merely the attachment of a 6-membered oxygen ring, and derivation of one of its isomers. Some preclinical data disclosed in the patent suggest that this series likely holds potential benefits over AZD5305.
This strategy of design is not uncommon, as evidenced by the KRAS inhibitor that Jinfang Pharmaceuticals transferred to Cinda. However, whether the advantages displayed in preclinical stages can effectively translate into clinical benefits is always filled with unknowns.




