Unleashing the Power of Raloxifene Hydrochloride: A Comprehensive Review on R&D Breakthroughs
Raloxifene Hydrochloride's R&D Progress
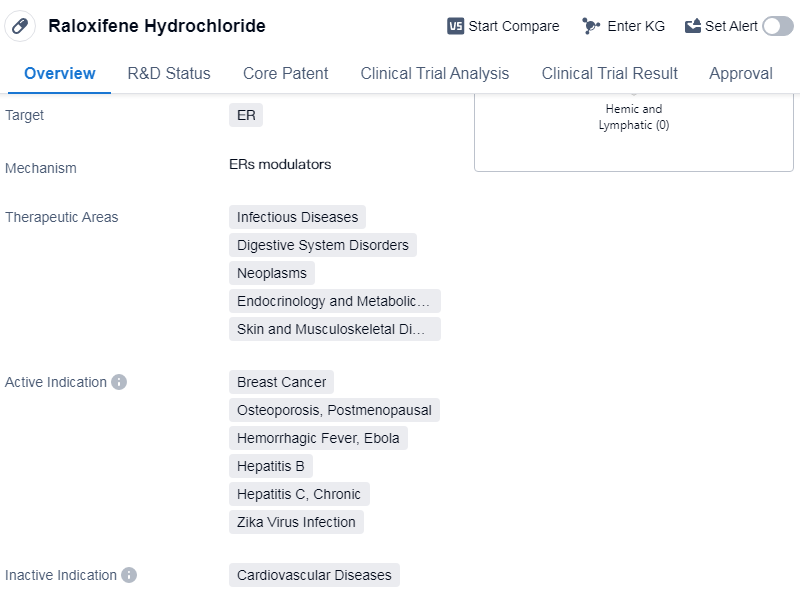
Raloxifene Hydrochloride is a small molecule drug that targets the estrogen receptor (ER). It is used in the treatment of various therapeutic areas including infectious diseases, digestive system disorders, neoplasms, endocrinology and metabolic diseases, as well as skin and musculoskeletal diseases. The drug has been approved for the treatment of breast cancer, osteoporosis in postmenopausal women, hemorrhagic fever caused by Ebola virus, chronic hepatitis B and C, and Zika virus infection.
The drug was developed by Eli Lilly & Co., a renowned pharmaceutical company. It received its first approval in the United States in December 1997. Raloxifene Hydrochloride has also obtained approvals in other countries, making it a globally recognized drug.
One notable aspect of Raloxifene Hydrochloride is its designation as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients. This designation provides certain incentives to the pharmaceutical company, such as market exclusivity and financial benefits, to encourage the development of drugs for rare diseases.
The approval of Raloxifene Hydrochloride for multiple indications highlights its versatility and potential in addressing various medical conditions. Breast cancer and osteoporosis are prevalent diseases, particularly in postmenopausal women, and the drug's approval for these indications signifies its efficacy in treating these conditions. Additionally, its approval for infectious diseases like hemorrhagic fever caused by Ebola virus, chronic hepatitis B and C, and Zika virus infection demonstrates its potential in combating these serious and often life-threatening conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Raloxifene Hydrochloride: ERs modulators
ERs modulators refer to substances or compounds that can interact with and modulate the activity of estrogen receptors (ERs). Estrogen receptors are proteins found in various tissues of the body, including the reproductive organs, bones, and brain. They play a crucial role in mediating the effects of estrogen, a hormone that regulates many physiological processes.
ERs modulators can have different effects on estrogen receptors depending on their specific properties. Some modulators may act as agonists, meaning they bind to the receptors and activate them, mimicking the effects of estrogen. These agonistic modulators can be used in hormone replacement therapy or to treat conditions related to estrogen deficiency.
On the other hand, some ERs modulators can act as antagonists, blocking the binding of estrogen to the receptors and inhibiting their activity. These antagonistic modulators are often used in the treatment of estrogen-dependent cancers, such as breast cancer, where reducing estrogen signaling is beneficial.
Additionally, there are selective ERs modulators (SERMs) that can have tissue-specific effects. These compounds selectively activate or inhibit estrogen receptors in specific tissues while having different effects in other tissues. SERMs are used in the treatment of conditions like osteoporosis and to reduce the risk of breast cancer in high-risk individuals.
Overall, ERs modulators are substances that can interact with estrogen receptors to either activate or inhibit their activity, providing a means to regulate estrogen signaling in various physiological and pathological conditions.
Drug Target R&D Trends for Raloxifene Hydrochloride
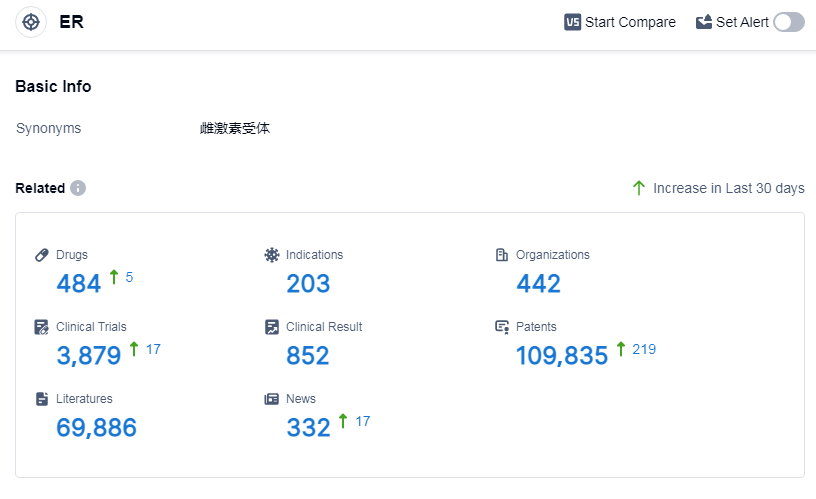
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 484 ER drugs worldwide, from 442 organizations, covering 203 indications, and conducting 3879 clinical trials.
The current competitive landscape for the target ER is characterized by the dominance of companies like Bayer AG, Pfizer Inc., and Mochida Pharmaceutical Co., Ltd. These companies have made significant progress in terms of drug development, with a focus on indications such as contraception, osteoporosis, and breast cancer. The development of small molecule drugs and PROTACs indicates intense competition and innovation in the field. The United States, China, and the European Union are leading in terms of drug development, with China showing notable progress. Overall, the target ER presents a promising future for the pharmaceutical industry, with potential advancements in treatment options for various indications.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Raloxifene Hydrochloride is a small molecule drug developed by Eli Lilly & Co. that targets the estrogen receptor. It has been approved for the treatment of breast cancer, osteoporosis, hemorrhagic fever caused by Ebola virus, chronic hepatitis B and C, and Zika virus infection. Its approval in multiple therapeutic areas and its designation as an orphan drug highlight its versatility and potential in addressing various medical conditions.