Pharmaceutical Insights: Retapamulin's R&D Progress and its Mechanism of Action on Drug Target
Retapamulin's R&D Progress
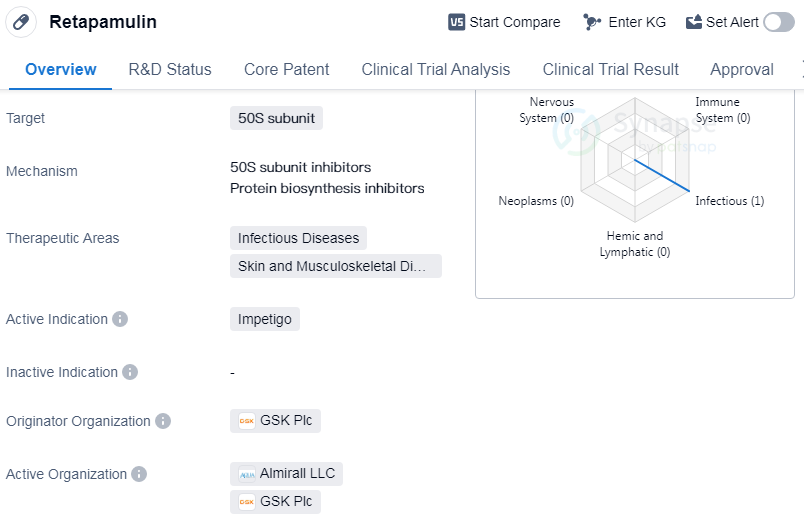
Retapamulin is a small molecule drug that targets the 50S subunit. It is primarily used in the treatment of infectious diseases, specifically impetigo, as well as skin and musculoskeletal diseases. The drug was first approved by the United States in April 2007.
Retapamulin was developed by GSK Plc, a leading pharmaceutical organization. As a small molecule drug, it is designed to interact with specific molecular targets in the body, in this case, the 50S subunit. This target is crucial for the survival and replication of bacteria, making it an ideal target for drugs aimed at treating infectious diseases.
The therapeutic areas of infectious diseases, skin, and musculoskeletal diseases highlight the potential applications of retapamulin. Impetigo, a highly contagious bacterial skin infection, is the active indication for this drug. By targeting the 50S subunit, retapamulin disrupts bacterial protein synthesis, ultimately leading to the inhibition of bacterial growth and the resolution of the infection.
The approval of retapamulin in 2007 marked a significant milestone in the pharmaceutical industry. It demonstrated the effectiveness and safety of the drug in treating impetigo, providing healthcare professionals with a new option for managing this common infectious disease. The United States was the first country to recognize the potential of retapamulin and grant approval, indicating the importance of this drug in the American healthcare system.
As a business development expert in the pharmaceutical industry, it is crucial to understand the key details of drugs like retapamulin. This information allows for informed decision-making regarding potential partnerships, licensing agreements, and market opportunities. The objective presentation of the drug's characteristics, including its drug type, targets, therapeutic areas, active indication, originator organization, and approval details, provides a comprehensive overview of retapamulin's profile.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Retapamulin: 50S Subunit Inhibitors and Protein Biosynthesis Inhibitors
50S subunit inhibitors are a type of drug that specifically targets the 50S subunit of the bacterial ribosome, which is involved in protein biosynthesis. These inhibitors work by binding to the 50S subunit and interfering with the normal function of the ribosome, ultimately preventing the synthesis of proteins in bacteria. By inhibiting protein biosynthesis, these drugs can effectively inhibit bacterial growth and replication, making them useful in the treatment of bacterial infections. Examples of 50S subunit inhibitors include macrolide antibiotics such as erythromycin, clarithromycin, and azithromycin. These drugs are commonly used to treat respiratory tract infections, skin infections, and certain sexually transmitted diseases caused by susceptible bacteria. It is important to note that 50S subunit inhibitors are specific to bacterial ribosomes and do not have the same effect on human ribosomes, making them selective in their antimicrobial activity.
Drug Target R&D Trends for Retapamulin
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 148 50S subunit drugs worldwide, from 159 organizations, covering 186 indications, and conducting 2218 clinical trials.
The analysis of the target 50S subunit reveals a competitive landscape with multiple companies focusing on its development. Pfizer Inc.leads in terms of the highest stage of development, with several approved drugs. Bacterial infection is the most common indication for drugs targeting the 50S subunit. Small molecule drugs are progressing rapidly, indicating intense competition. China, the United States, and Japan are the countries/locations with the fastest development, with China showing significant progress. Overall, the target 50S subunit presents opportunities for further research and development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Retapamulin is a small molecule drug developed by GSK Plc that targets the 50S subunit. It is primarily used in the treatment of impetigo, an infectious disease, as well as skin and musculoskeletal diseases. The drug was first approved by the United States in 2007. This information serves as a foundation for further analysis and evaluation of retapamulin's potential in the pharmaceutical market.