Patient Receives First Dose of ALPK1 Inhibitor, DF-003, in Drug Farm's Phase 1 Clinical Trial
Drug Farm, currently in the clinical phase, has shared the news of the first participant in the Phase 1 clinical experiment, testing DF-003, receiving their initial dosage. This experimental drug, DF-003, found utilizing Drug Farm’s distinct MedChem5 and ID In Vivo technologies, is a small molecule substance that puts a stop to alpha-kinase 1 (ALPK1).
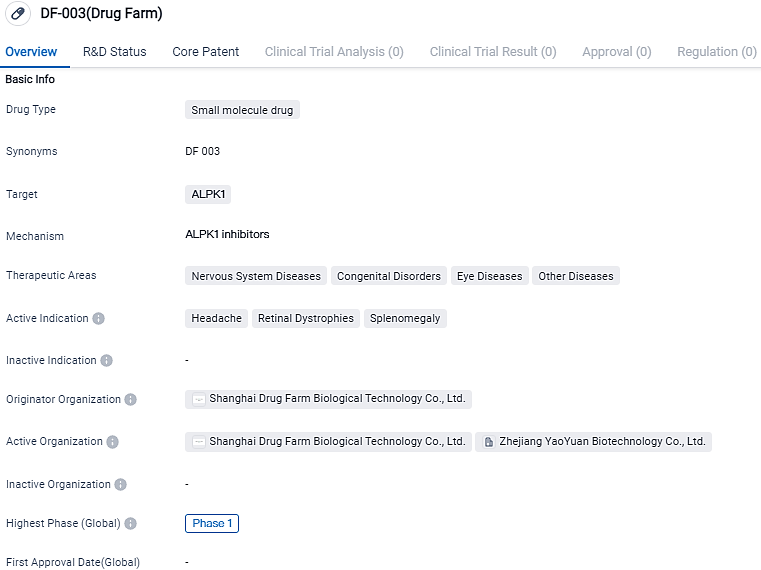
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
ALPK1 is a crucial element that manipulates inflammation pathways connected to disease origin in cardio-renal issues. Furthermore, a dramatic increase in the functionality of ALPK1 provokes a seldom found genetic condition known as ROSAH syndrome, leading to a loss of sight.
Dr. Tony Xu, Ph.D., co-founder and Chief Operating Officer of Drug Farm, expressed enthusiasm over the upcoming research resulting from their technological advancements, particularly regarding their first-class drug, DF-003. This drug, being an orally administerable, robust and uniquely selective ALPK1 inhibitor, has shown potential in preliminary trials in cardio-renal and ROSAH disease animal models.
The Phase 1 study of DF-003 will involve healthy volunteers testing single and multiple increasing dosages, a process that will guide the dosage recommendations for Phase 2 programs. Dr. Jeysen Yogaratnam, MB.BCh, MRCSEd, Ph.D., MBA, the Chief Medical Officer at Drug Farm, anticipates wrapping up the Phase 1 study and eventually offering a novel treatment for those suffering from heart and kidney diseases, including ROSAH syndrome.
Drug Farm is pioneering novel therapies primarily aimed at innate immunity in conditions like hepatitis B, heart and kidney diseases, and ROSAH syndrome. Utilizing their distinct IDInVivo platform, which amalgamates revolutionary genetic and AI technologies, they are able to uncover new treatments. Drug Farm has pinpointed fresh innate immunity pathways and targets, and is promptly progressing multiple novel first-in-class drug candidates towards clinical trials.
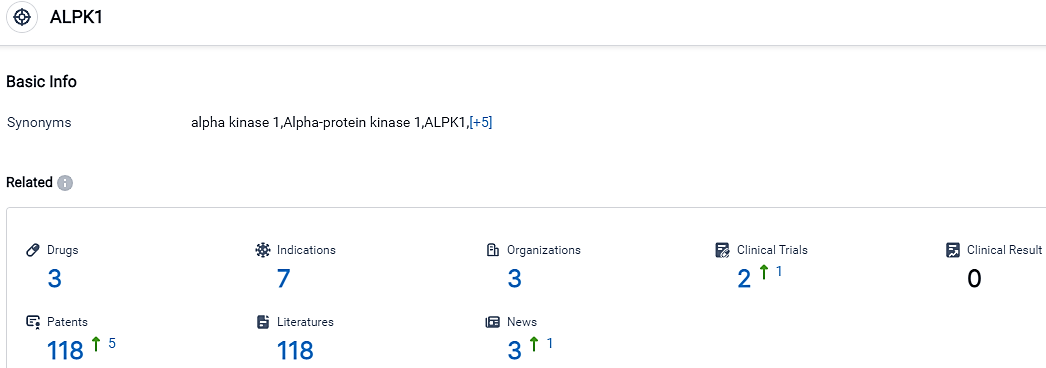
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 22, 2023, there are 3 investigational drugs for the ALPK1 target, including 7 indications,3 R&D institutions involved, with related clinical trials reaching 2,and as many as 118 patents.
DF-003 is a small molecule drug developed by Shanghai Drug Farm Biological Technology Co., Ltd. It targets ALPK1 and has shown potential in treating various diseases, including Nervous System Diseases, Congenital Disorders, Eye Diseases, and Other Diseases. The drug is currently in Phase 1, the highest phase of development globally, and its successful progression through this phase will determine its future potential as a therapeutic option.