The Abelacimab AF study was early terminated by the Data Monitoring Committee due to a major decline in bleeding compared to a DOAC
Blackstone Life Sciences-backed Anthos Therapeutics, Inc., a firm actively engaged in the development of novel treatments for heart diseases, declared that their AZALEA-TIMI 71 Phase 2 trial involving 1,287 patients suffering from atrial fibrillation and having a medium to high stroke risk, achieved its primary endpoint.
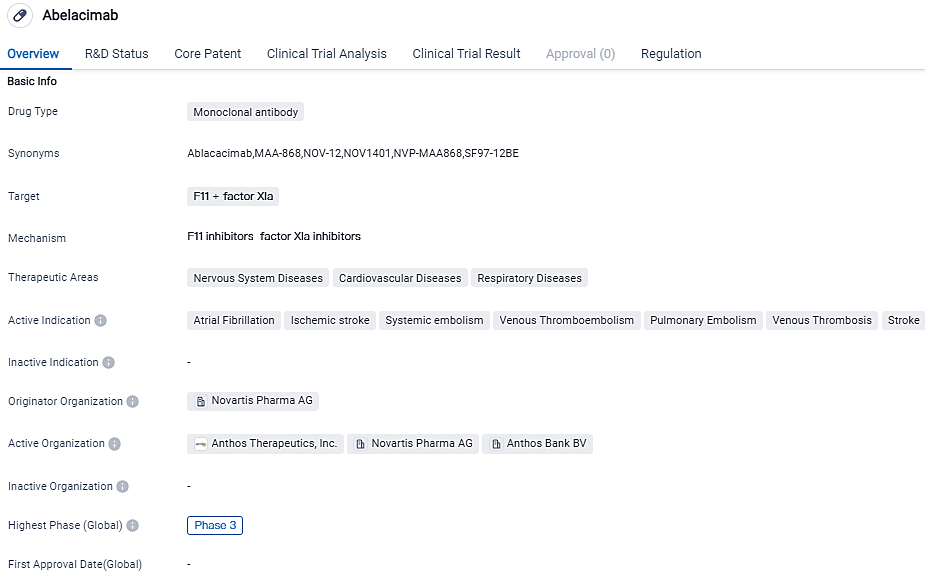
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The Data Monitoring Committee prematurely halted the research due to a significant decrease in the combination of serious and clinically significant less severe bleeding in those administered with abelacimab versus others on rivaroxaban, a dominant standard-of-care DOAC. Also, abelacimab is the pioneering and sole Factor XI inhibitor to prove a previously unseen lowering in major bleeding relative to a DOAC, the gravest category of bleeding. The comprehensive findings of AZALEA-TIMI 71 will be disclosed at an approaching scientific conference.
Marc S. Sabatine, MD, MPH, the Lewis Dexter, MD, Distinguished Chair in Cardiovascular Medicine commented: "The AZALEA-TIMI 71 examination is the most extensive and longest comparative study of a Factor XI inhibitor and offers irrefutable evidence of a key reduction in bleeding contrasted with the standard-of-care anticoagulant. The review of AZALEA-TIMI 71 concludes a critical study affirming the potential of Factor XI inhibition for inflicting considerably less bleeding than the prevailing standard-of-care."
Dan Bloomfield, MD, Chief Medical Officer of Anthos Therapeutics stated: "Given the impressive decrease in bleeding shown by the AZALEA-TIMI 71 study, coupled with an 80% decrease in thrombosis in our previous VTE research, abelacimab proves its potential as a haemostasis-friendly anticoagulant and signifies a major alteration in stroke and other thrombotic conditions prevention methods. If approved, more patients with atrial fibrillation could be treated effectively and safely, with a much lower risk of bleeding with abelacimab as compared to a DOAC."
CDC estimates that the United States will have 12.1 million people suffering from atrial fibrillation by 2030. Unfortunately, anticoagulants are not prescribed for 40% to 60% of patients with atrial fibrillation. Anthos Therapeutics has started an extension study allowing patients to switch from rivaroxaban to abelacimab to take advantage of the enhanced bleeding profile. More so, a Fast-Track Designation for abelacimab was previously given by the U.S. FDA specifically for averting stroke and systemic embolism in patients with atrial fibrillation.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
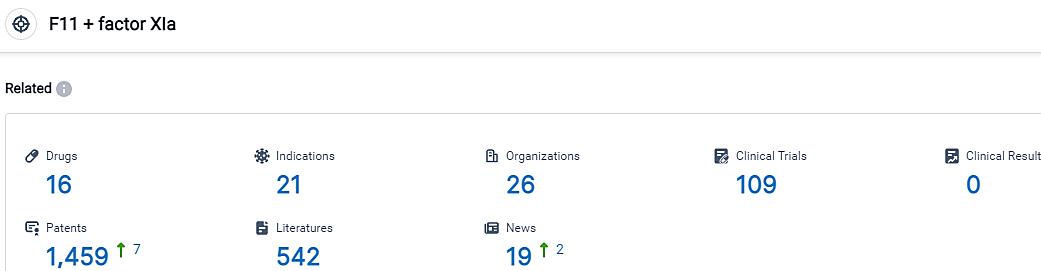
According to the data provided by the Synapse Database, As of September 22, 2023, there are 16 investigational drugs for the F11 and factor XIa target, including 21 indications,26 R&D institutions involved, with related clinical trials reaching 109,and as many as 1459 patents.
Abelacimab is a novel, highly selective, fully human monoclonal antibody that locks Factor XI in the inactive state, resulting in dual inhibitory activity against both Factor XI and its activated form.
Factor XI inhibition offers the promise of hemostasis-sparing anticoagulation for the prevention and treatment of arterial and venous thromboembolic events. Abelacimab is an investigational agent and is not approved for any indication in any country.