Pfizer Progresses With Daily Dosage Version of Oral GLP-1 Receptor Agonist Danuglipron
Pfizer Inc. revealed that, according to the findings from the ongoing pharmacokinetic research, they have chosen the preferred once-daily modified release form of danuglipron, an oral glucagon-like peptide-1 (GLP-1) receptor agonist. The company intends to carry out dose optimization studies in the latter half of 2024 to evaluate various doses of the selected modified release formulation, which will support the registration enabling studies.
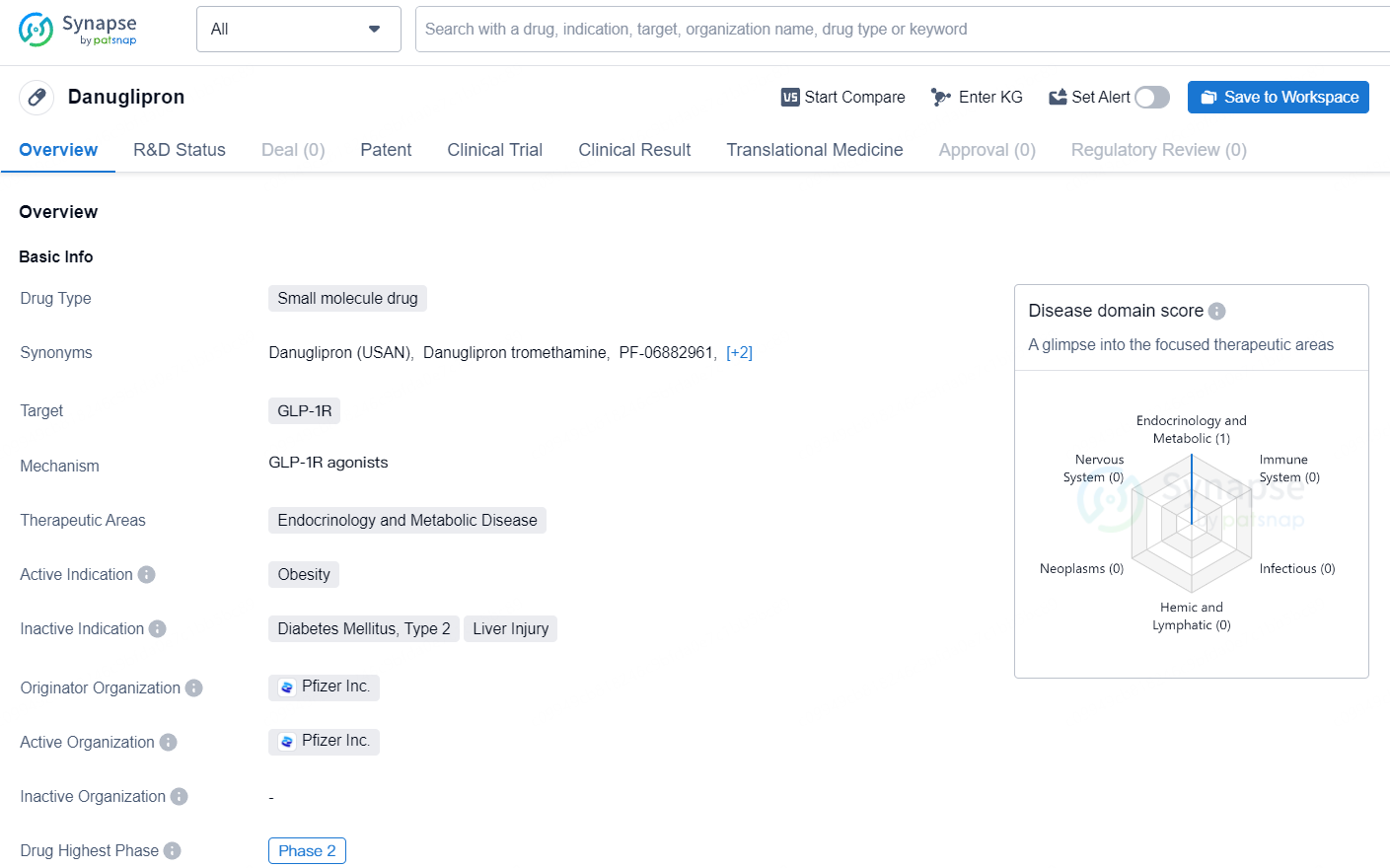
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Obesity is a crucial therapeutic focus area for Pfizer, and the company possesses a significant portfolio of three clinical and numerous pre-clinical candidates. The leading candidate, danuglipron, has exhibited strong efficacy in a twice-daily regimen. There is potential for a once-daily formulation to offer a competitive advantage in the oral GLP-1 market, as noted by Mikael Dolsten, MD., PhD., Chief Scientific Officer and President of Pfizer Research and Development.
"Through an in-depth review of our earlier Phase 2b data and the study design, we believe that with an optimized modified release formulation and improved future trial designs, we can progress a competitive oral GLP-1 candidate to registration-enabling studies. Our objective is to meet the ongoing and significant medical needs of individuals managing obesity," Mikael Dolsten further stated.
An ongoing open-label, randomized investigation is assessing the pharmacokinetics and safety of both immediate and modified release formulations of danuglipron when taken orally by healthy adults aged 18 or older. Thus far, the study has shown results indicating a pharmacokinetic profile supportive of once-daily dosing, with a safety profile consistent with previous danuglipron investigations, including no observed liver enzyme elevations among over 1,400 participants
Danuglipron (PF-06882961) is an experimental medication administered orally as a tablet and is currently not approved by regulatory authorities. Discovered and developed internally at Pfizer, danuglipron is known as a GLP-1 receptor agonist. This investigational drug aims to maintain blood glucose at healthy levels by enhancing insulin secretion. Additional effects may include slowing gastric emptying and increasing satiety post-meal, potentially contributing to weight reduction.
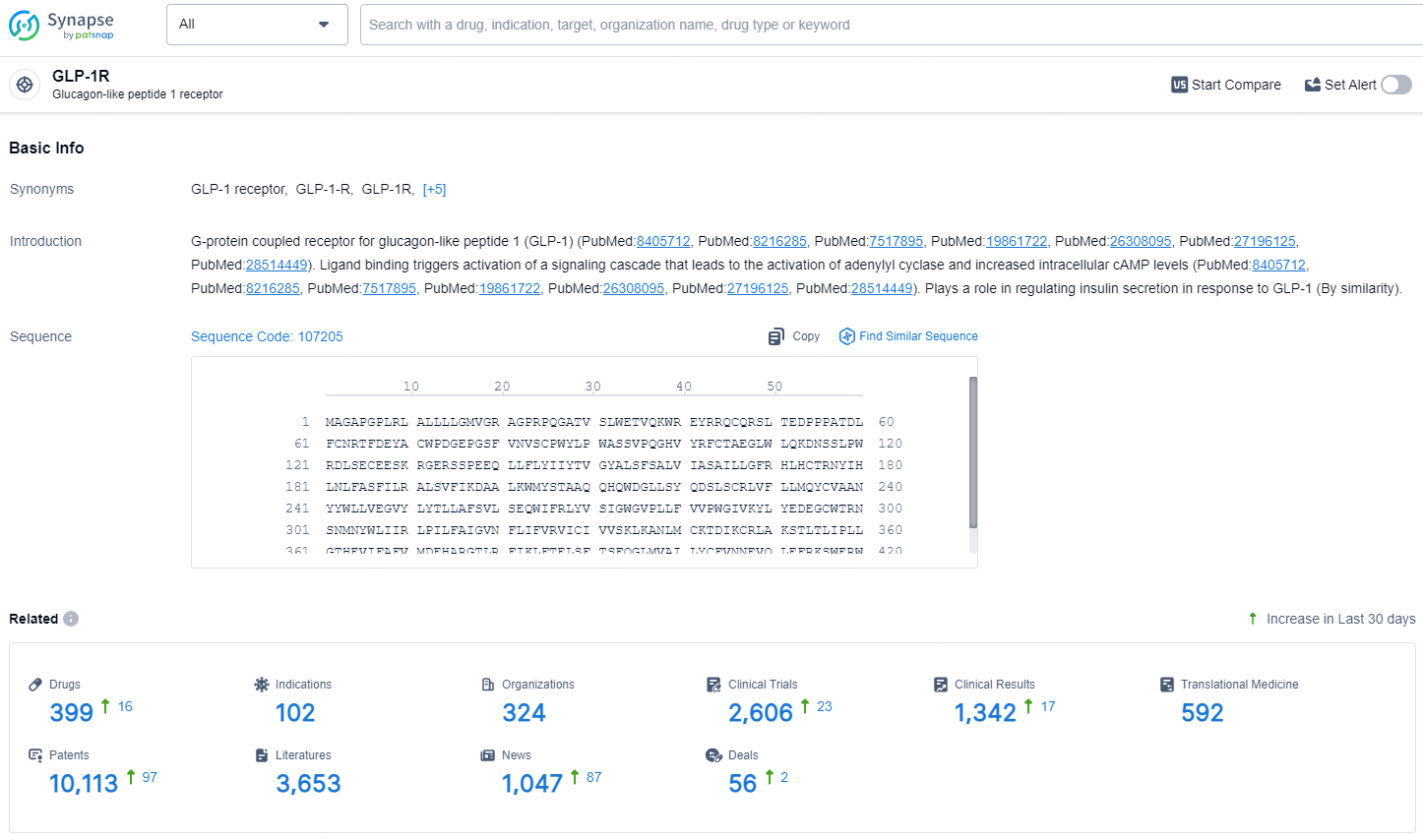
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 16, 2024, there are 399 investigational drugs for the GLP-1 target, including 102 indications, 324 R&D institutions involved, with related clinical trials reaching 2606, and as many as 10113 patents.
Danuglipron is a small molecule drug that targets the GLP-1 receptor, primarily focusing on therapeutic areas related to endocrinology and metabolic diseases, with an active indication in treating obesity. The drug has reached the highest phase of development globally, which is Phase 2.