Phase 3 SELECT-GCA Study: Upadacitinib Shows Promise in Treating Giant Cell Arteritis
AbbVie declared successful preliminary outcomes from its SELECT-GCA clinical trial, a Phase 3 study that was multicenter, randomized, double-blind, and placebo-controlled. The study demonstrated that upadacitinib (RINVOQ®; 15 mg, once daily) alongside a steroid tapering program over 26 weeks met the primary goal of maintaining remission from week 12 to week 52 in adult patients suffering from giant cell arteritis.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In this investigation, 46% of participants treated with 15 mg upadacitinib alongside a 26-week steroid tapering protocol achieved lasting remission. This contrasts with a 29% remission rate in patients who were given a placebo within a 52-week steroid taper schedule.
"Numerous individuals affected by GCA still experience its severe symptoms, facing few available therapeutic alternatives," noted Kori Wallace, M.D., Ph.D., Vice President and Global Head of Immunology Clinical Development at AbbVie. "These findings underscore our unwavering dedication to enhancing the quality of life for those suffering from immune-mediated conditions through the advancement of novel therapies where substantial healthcare demands persist."
GCA, an autoimmune disorder, leads to the inflammation of the temporal and other cranial arteries, the aorta, and other large to medium-sized arteries. Predominantly, GCA affects individuals over 50 years, with those between 70 to 80 years experiencing it most frequently. Females are more susceptible to this condition, which can result in symptoms like headaches, jaw pain, and vision alterations or loss, including acute and irreversible vision loss.
"These findings are promising, and they contribute to the existing data supporting the effectiveness and safety of using upadacitinib in treating rheumatic conditions," stated Daniel Blockmans, M.D., Ph.D., from the Department of General Internal Medicine at University Hospitals Gasthuisberg, Belgium, also a professor of medicine at KU Leuven, Belgium, and the primary investigator of the SELECT-GCA study.
"Given these outcomes, upadacitinib could emerge as the inaugural oral medication option for GCA patients. It targets the inflammation in large arteries chiefly affecting the elderly and currently has only one formally sanctioned treatment that is typically administered with steroids."
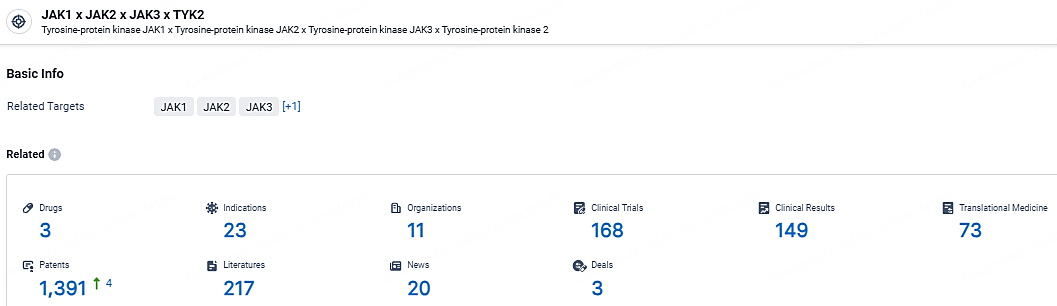
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 22, 2024, there are 3 investigational drugs for the JAK1 and JAK2 and JAK3 and TYK2 target, including 23 indications, 11 R&D institutions involved, with related clinical trials reaching 168, and as many as 1391 patents.
Upadacitinib is a small molecule drug that targets multiple JAK enzymes. It has been approved for various indications across different therapeutic areas. Its first approval was granted in the United States in August 2019, and it has undergone regulatory processes such as priority review, orphan drug designation, and special review projects. Upadacitinib shows promise in addressing a wide range of diseases and has the potential to make a significant impact in the field of biomedicine.