Phase II Trial Begins for ABY-025 PET Diagnostic in Gastric and Esophageal Cancers
Swedish biotech firm Affibody AB has disclosed the commencement of treatment for the inaugural group of individuals diagnosed with gastroesophageal malignancy participating in a Phase 2 clinical basket trial. This study is focused on evaluating the effectiveness of the novel PET tracer, 68Ga-ABY-025, which is utilized for the non-invasive measurement of HER2 expression across various solid neoplasms. The therapeutic agent ABY-025 relies on the unique properties of an Affibody molecule, which exhibits a robust affinity for the HER2 receptor, a protein found on the cell membrane that is involved in the progression of multiple cancer types. The significant binding strength to HER2 coupled with the swift elimination of ABY-025 from both blood and healthy tissues enables a swift determination of HER2 levels within a few hours post-administration.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The fight against cancers of the stomach and esophagus represents a significant international health issue, particularly when it comes to early identification and the implementation of successful therapeutic strategies. The medical field is in urgent demand for less invasive diagnostic methods that are capable of informing the development of new treatments. There is considerable optimism surrounding the progress of therapies targeting HER2 for these cancer patients.
Fredrik Frejd, a Professorial expert, and Chief Scientific Officer at Affibody, reflected on the progress made within the ABY-025 initiative, specifically pointing to its expanded application in the battle against stomach and esophageal cancers. He expressed his gratitude for the collaborative efforts with esteemed colleagues at the Karolinska Institute and Karolinska University Hospital. Frejd believes that the findings from ongoing studies will significantly bolster the potential of their therapeutic agent ABY-271 for individuals afflicted by these cancers.
The clinical study, integral to Affibody's radiopharmaceutical endeavors, seeks to evaluate the utility of 68Ga-ABY-025 in the non-invasive assessment of HER2 expression levels within various solid tumors. Recently, researchers released data concerning a subset of breast cancer subjects marked by reduced HER2 levels. The study is now shifting to recruit and incorporate subjects diagnosed with stomach and esophageal cancers.
As noted by Rimma Axelsson, a distinguished Professor in Nuclear Medicine and the head researcher of this initiative, recent investigations revealed the potential of 68Ga-ABY-025 to detect metastatic sites expressing low HER2 levels in breast cancer patients. Axelsson is looking forward to broadening this research to include more individuals.
Magnus Nilsson, a Professor of Surgical practices and co-research lead, highlighted the inconsistencies in HER2 expression levels among varying lesions in patients with stomach and esophageal cancers. He stressed the importance of accurately determining the status of HER2 receptors in these patients to effectively direct therapy tailored toward HER2.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
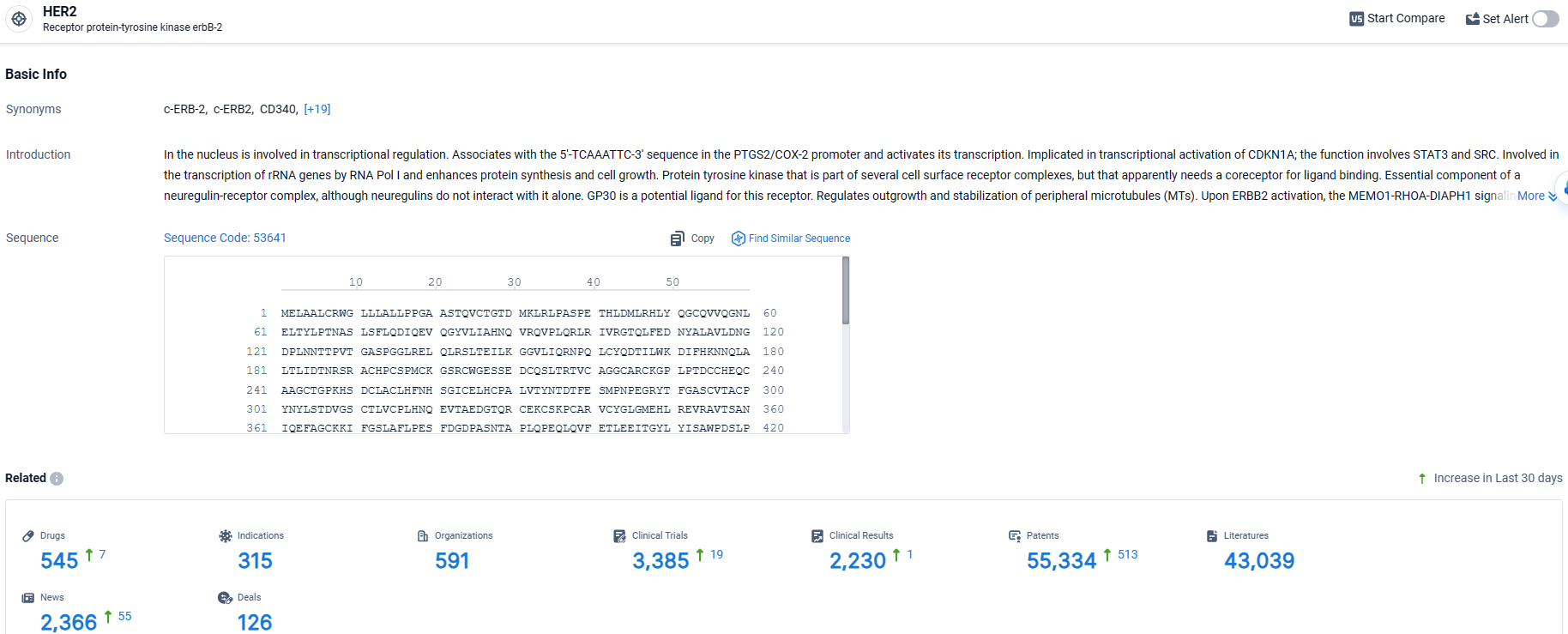
According to the data provided by the Synapse Database, As of March 27 2024, there are 545 investigational drugs for the HER2 target, including 315 indications, 591 R&D institutions involved, with related clinical trials reaching 3385, and as many as 55334 patents.
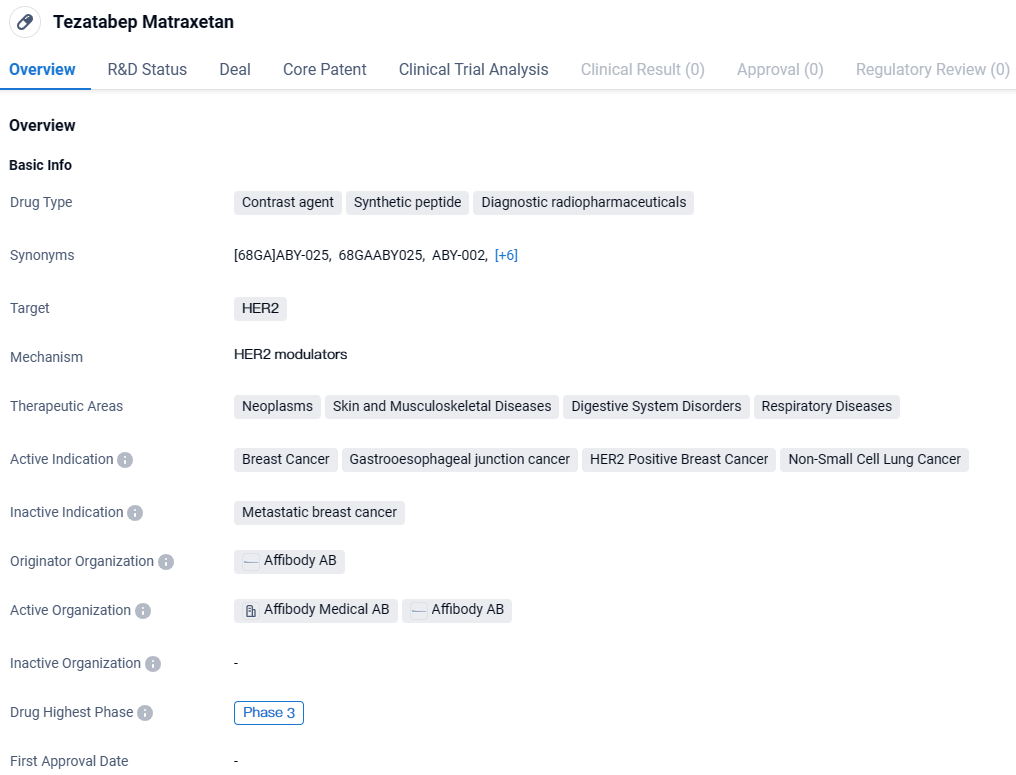
Tezatabep Matraxetan is a contrast agent and synthetic peptide that belongs to the category of diagnostic radiopharmaceuticals. It targets HER2 and has shown potential in the treatment of various cancers, including breast cancer, gastroesophageal junction cancer, HER2 positive breast cancer, and non-small cell lung cancer. The drug is developed by Affibody AB and has reached Phase 3 of clinical development, indicating its advanced stage of evaluation.