Preliminary Clinical Results for TYRA-300, a Selective FGFR3 Inhibitor by Tyra Biosciences
Tyra Biosciences, Inc. (Nasdaq: TYRA), a biotechnology firm in the clinical stage, is dedicated to creating advanced precision therapies that specifically address significant opportunities in the field of Fibroblast Growth Factor Receptor (FGFR) biology. The company announced that it has obtained clinical proof-of-concept data for TYRA-300 from its ongoing SURF301 Phase 1/2 trial involving patients with metastatic urothelial (mUC) cancer. These findings will be shared during a late-breaking oral session at the 36th EORTC-NCI-AACR (ENA) Symposium on Molecular Targets and Cancer Therapeutics, scheduled for October 23-25, 2024, in Barcelona, Spain. TYRA-300 represents a promising potential first-in-class, investigational, oral inhibitor that selectively targets FGFR3, aiming to minimize the toxic effects linked to the inhibition of FGFR1, FGFR2, and FGFR4, while remaining unresponsive to FGFR3 gatekeeper mutations.
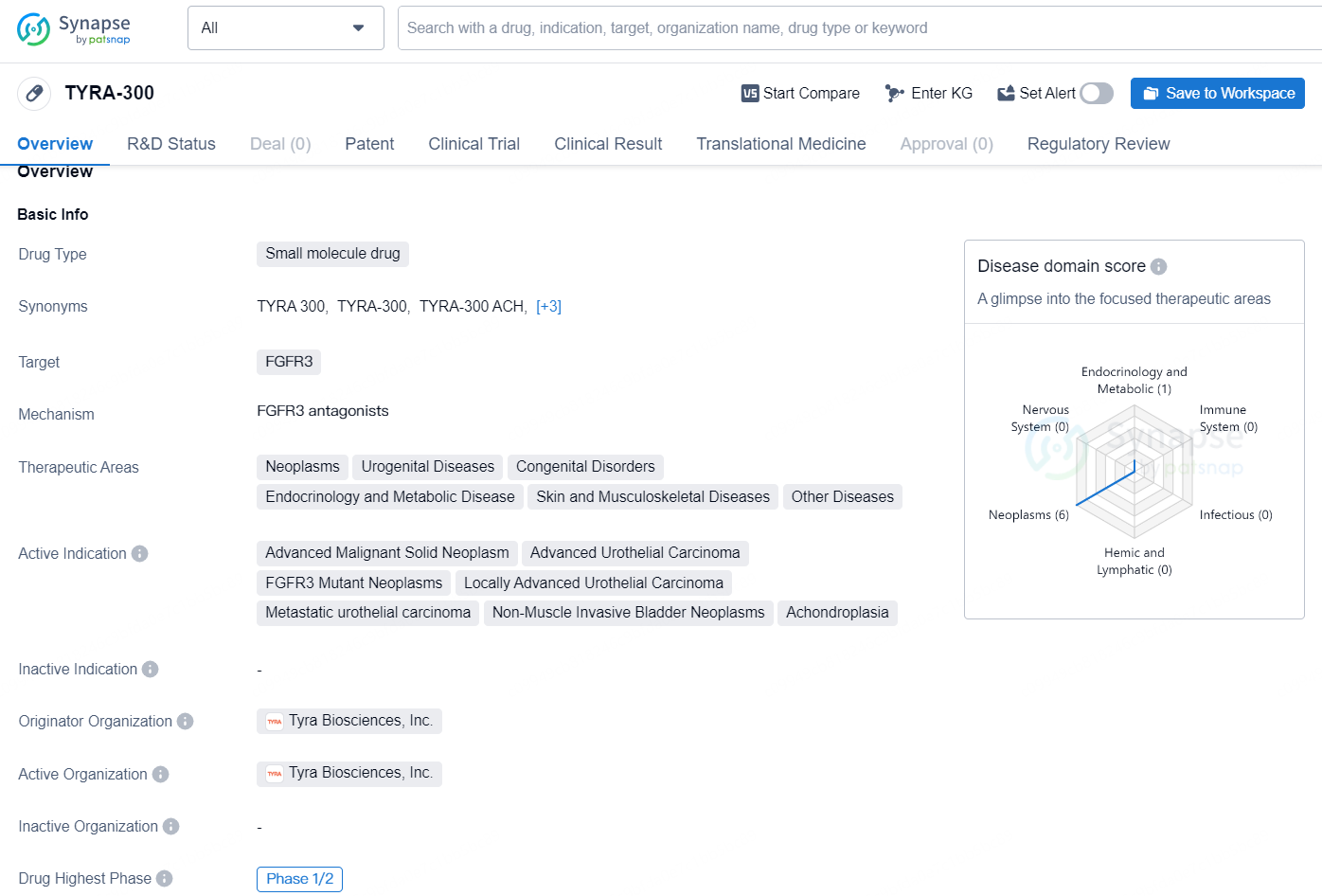
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“Alterations in FGFR3 are recognized as a key factor influencing tumor behavior in a specific group of urothelial cancer cases. Although pan-FGFR inhibitors have shown effectiveness and received approval for treating FGFR3-altered urothelial cancer, their use is often limited by various severe on-target side effects. There is an ongoing need to provide enhanced precision treatment for patients with urothelial cancer, enabling them to not only prolong their lives but also improve their quality of life,” stated Dr. Ben Tran, Associate Professor at the Peter MacCallum Cancer Centre in Melbourne, Australia. “The preliminary findings from the TYRA-300 trial are very promising. I believe TYRA-300 has the potential to emerge as a next-generation targeted treatment that exhibits high selectivity for FGFR3. This early evidence suggests that TYRA-300 can offer better anti-tumor efficacy and tolerability for patients with FGFR3-altered urothelial cancer. TYRA-300 holds significant promise for enhancing patient outcomes, and I am eager to see its further development across all cancers with FGFR3 alterations.”
Interim Clinical Results Summary
As of August 15, 2024, the cutoff for data collection, 41 patients were enrolled in the Phase 1 segment of the SURF301 Phase 1/2 trial. Participants included adults with advanced cancers, both with and without FGFR3 alterations, including those who had previously been treated with erdafitinib. The enrolled cohort was extensively pre-treated, with 44% having undergone three or more lines of prior therapy before receiving TYRA-300, and 76% of FGFR3+ metastatic urothelial cancer (mUC) patients also having received three or more therapy lines. The treatment of TYRA-300 was assessed across six dose ranges, from 10 mg to 120 mg once daily (QD).
Initial pharmacokinetic/pharmacodynamic (PK/PD) analyses of the 41 patients by the cutoff date revealed that plasma concentrations of TYRA-300 indicated sufficient target coverage at a dose of ≥ 90 mg QD, with further PK investigations continuing.
In FGFR3+ mUC patients who were administered doses of ≥ 90 mg QD, all exhibited anti-tumor effects:
- 6 out of 11 (54.5%) patients at ≥ 90 mg QD reached a partial response (PR), with three still ongoing.
- 5 out of 10 (50%) patients at 90 mg QD also achieved a PR.
- A 100% disease control rate (DCR), which includes both PR and stable disease, was recorded among all patients receiving ≥ 90 mg QD.
As of the data cutoff, TYRA-300's interim safety profile appeared favorable:
Preliminary findings from SURF301 indicate that TYRA-300 was generally well-tolerated, with rare instances of toxicities associated with FGFR2 and FGFR1.
Among doses ranging from 10 mg to 120 mg QD, there were four serious adverse events (10%) connected to TYRA-300, one dose-limiting toxicity (DLT) of grade 3 diarrhea at a dose of 90 mg QD, and one treatment-related adverse event (TRAE) that necessitated treatment discontinuation (grade 3 ALT, 90 mg QD).
No TRAE of grade 4 or higher were reported.
The 120 mg QD dose was the maximum assessed, with no DLTs observed.
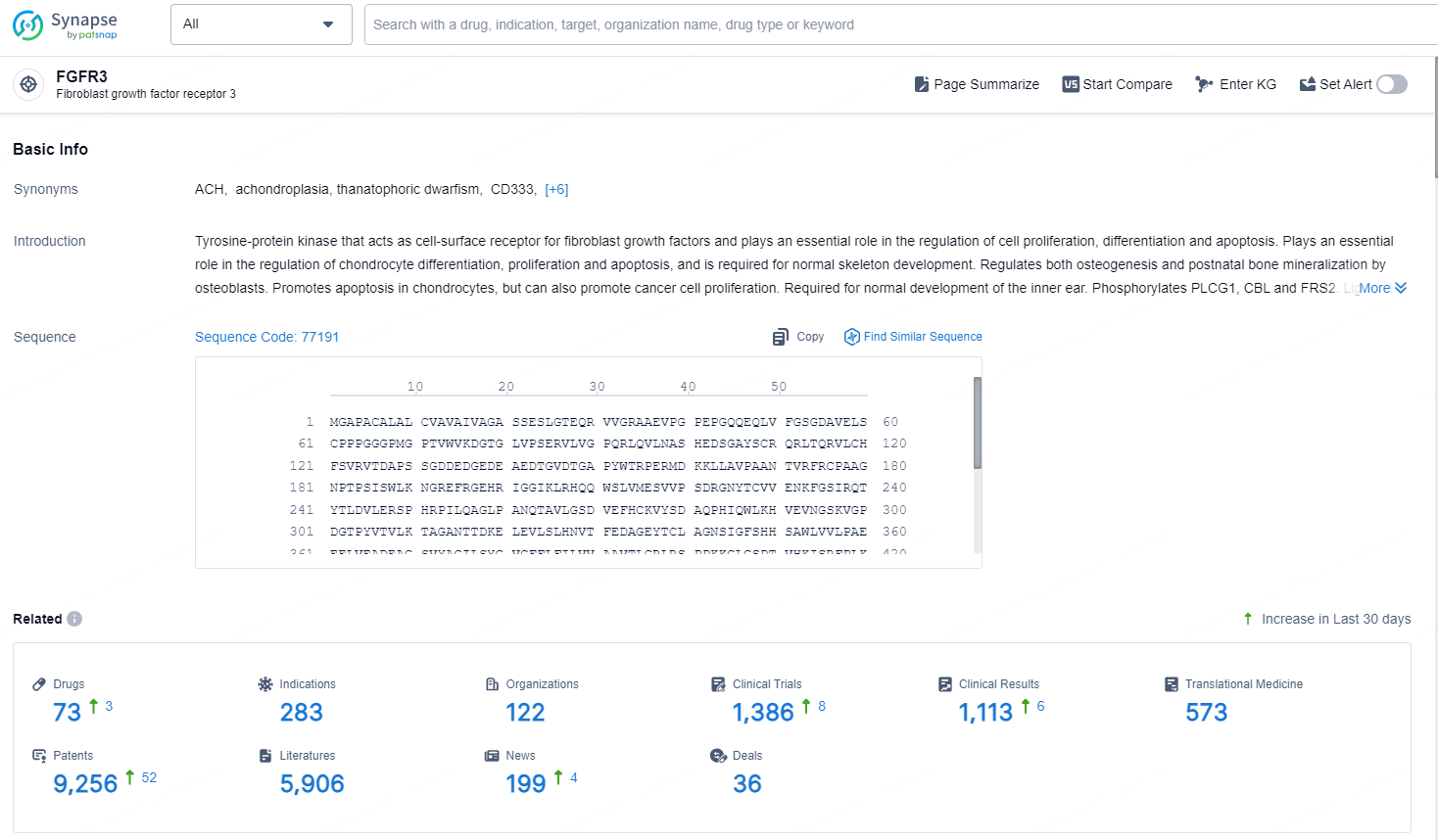
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of October 29, 2024, there are 73 investigational drugs for the FGFR3 target, including 283 indications, 122 R&D institutions involved, with related clinical trials reaching 1386, and as many as 9256 patents.
TYRA-300 is a small molecule drug developed by Tyra Biosciences, Inc. It targets FGFR3 and is intended to treat a wide range of therapeutic areas including neoplasms, urogenital diseases, congenital disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, and other diseases. The drug's active indications include advanced malignant solid neoplasm, advanced urothelial carcinoma, FGFR3 mutant neoplasms, locally advanced urothelial carcinoma, metastatic urothelial carcinoma, non-muscle invasive bladder neoplasms, and achondroplasia.