Decoding Infigratinib: a comprehensive study of its R&D trends and its clinical results in 2024 ASCO_GU
In the subset of patients with UC bearing alterations in the fibroblast growth factor 3 (FGFR3) gene, targeted therapies directed at this moiety have shown substantial antitumor effect in the metastatic setting. The 2024 ASCO_GU reported the latest clinical trial results of infigratinib, a potent and selective inhibitor of FGFR3, illustrating its potential clinical benefits.
Infigratinib's R&D Progress
Infigratinib is a small molecule drug that targets FGFR1, FGFR2, FGFR3, and FGFR4. The drug has shown potential therapeutic applications in various areas of biomedicine. These include neoplasms (abnormal growth of cells), congenital disorders (conditions present at birth), endocrinology and metabolic diseases, skin and musculoskeletal diseases, digestive system disorders, and nervous system diseases.
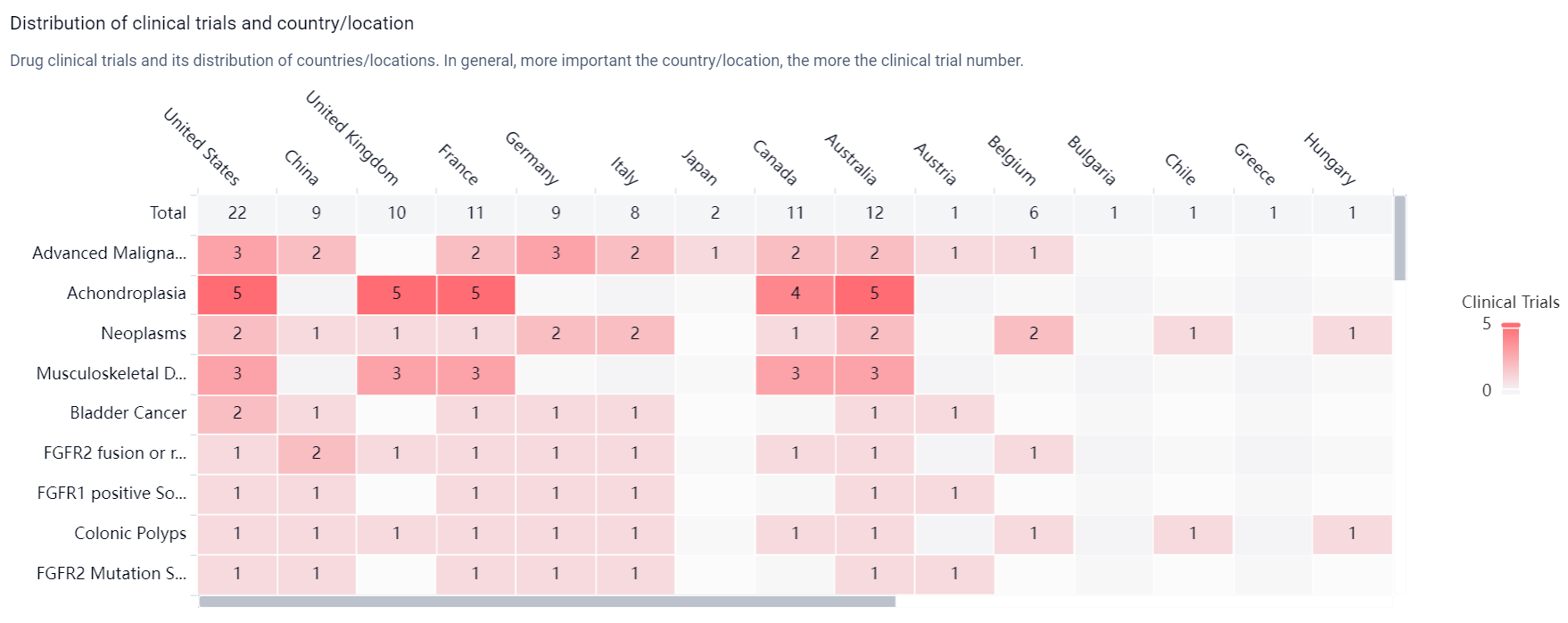
According to the Patsnap Synapse, Infigratinib is being developed by QED Therapeutics, Inc. and has reached the highest phase of clinical development, Phase 3, globally. In China, it has reached Phase 2. And the clinical trial distributions for Infigratinib are primarily in the United States, China and United Kingdom. The key indication is Advanced Malignant Solid Neoplasm.
Detailed Clinical Result of Infigratinib
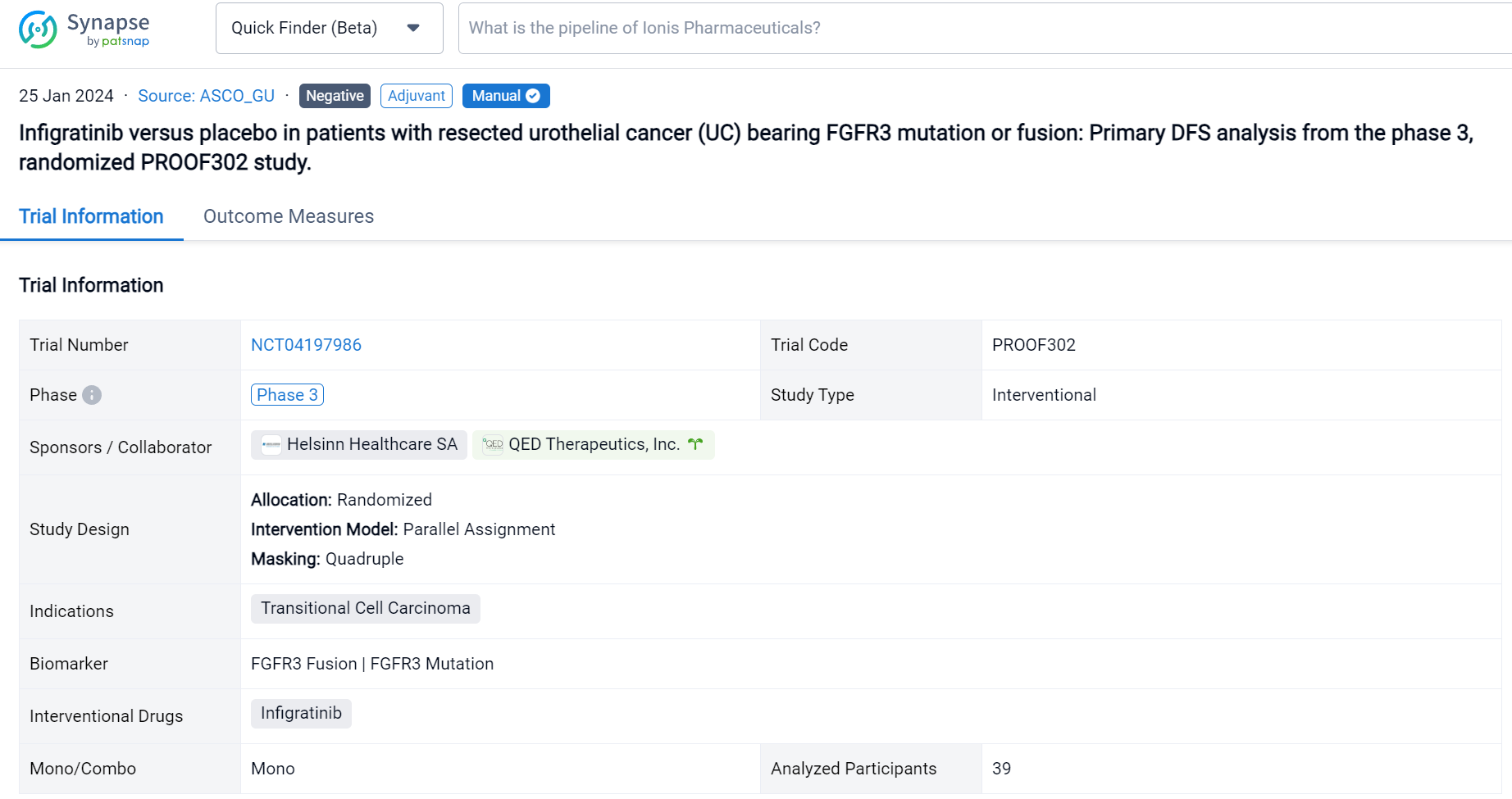
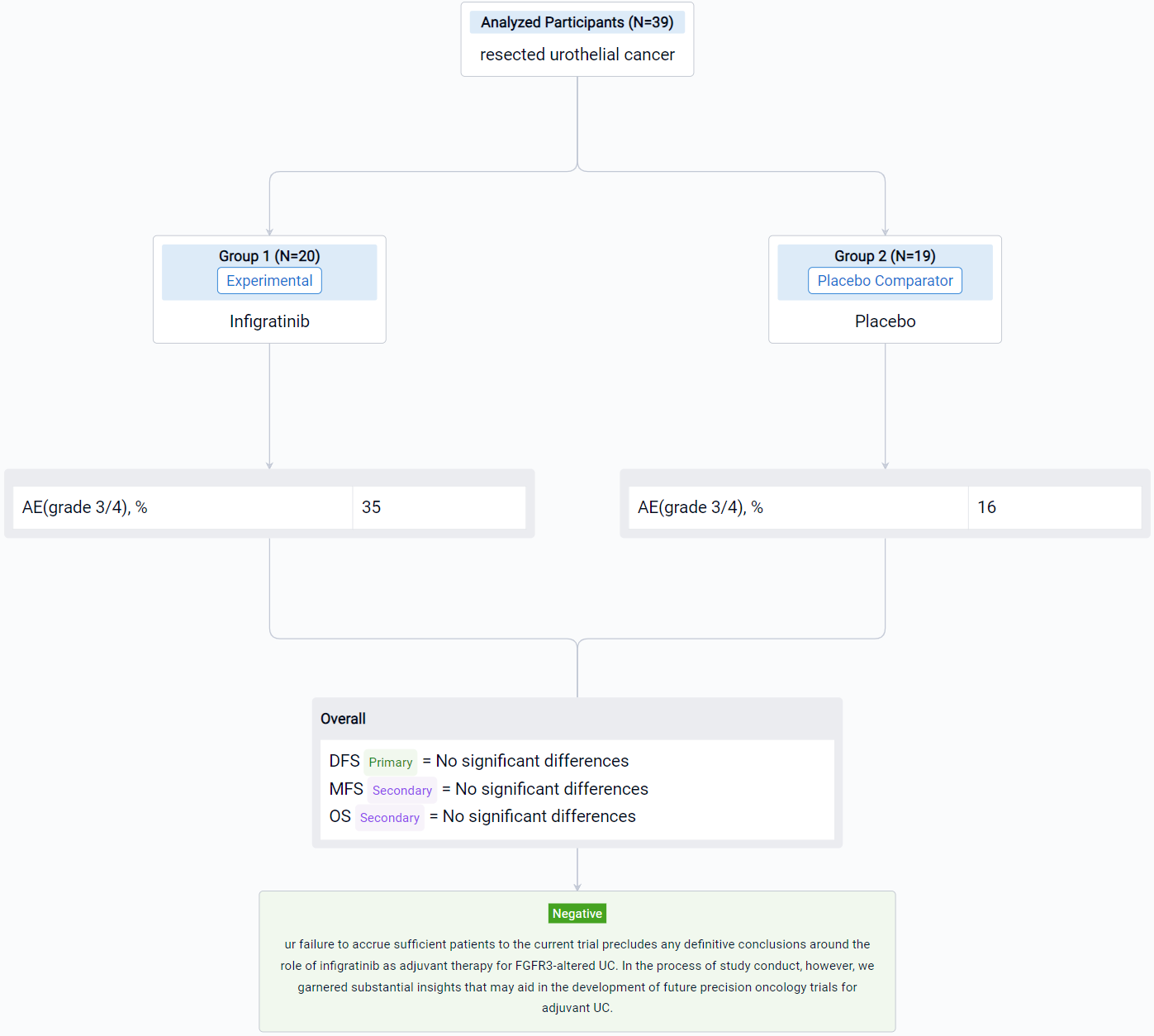
This randomized, parallel Assignment, Quadruple-blind clinical trial (NCT04197986) was aimed to evaluate the safety and efficacy of infigratinib as a adjuvant therapy in patients with high-risk resected UC.
In this study, the authors randomly assigned patients in a 1:1 ratio to receive either oral infigratinib (125 mg) or placebo daily for 21 days of a 28-day treatment cycle, for a maximum of 13 cycles or until disease recurrence. Eligible patients had confirmed invasive UC with susceptible FGFR3 alterations and had undergone radical surgery within 120 days of randomization. The primary endpoint of the study was centrally assessed disease-free survival (DFS), with secondary endpoints including investigator-assessed DFS, metastasis-free survival (MFS) and overall survival (OS).
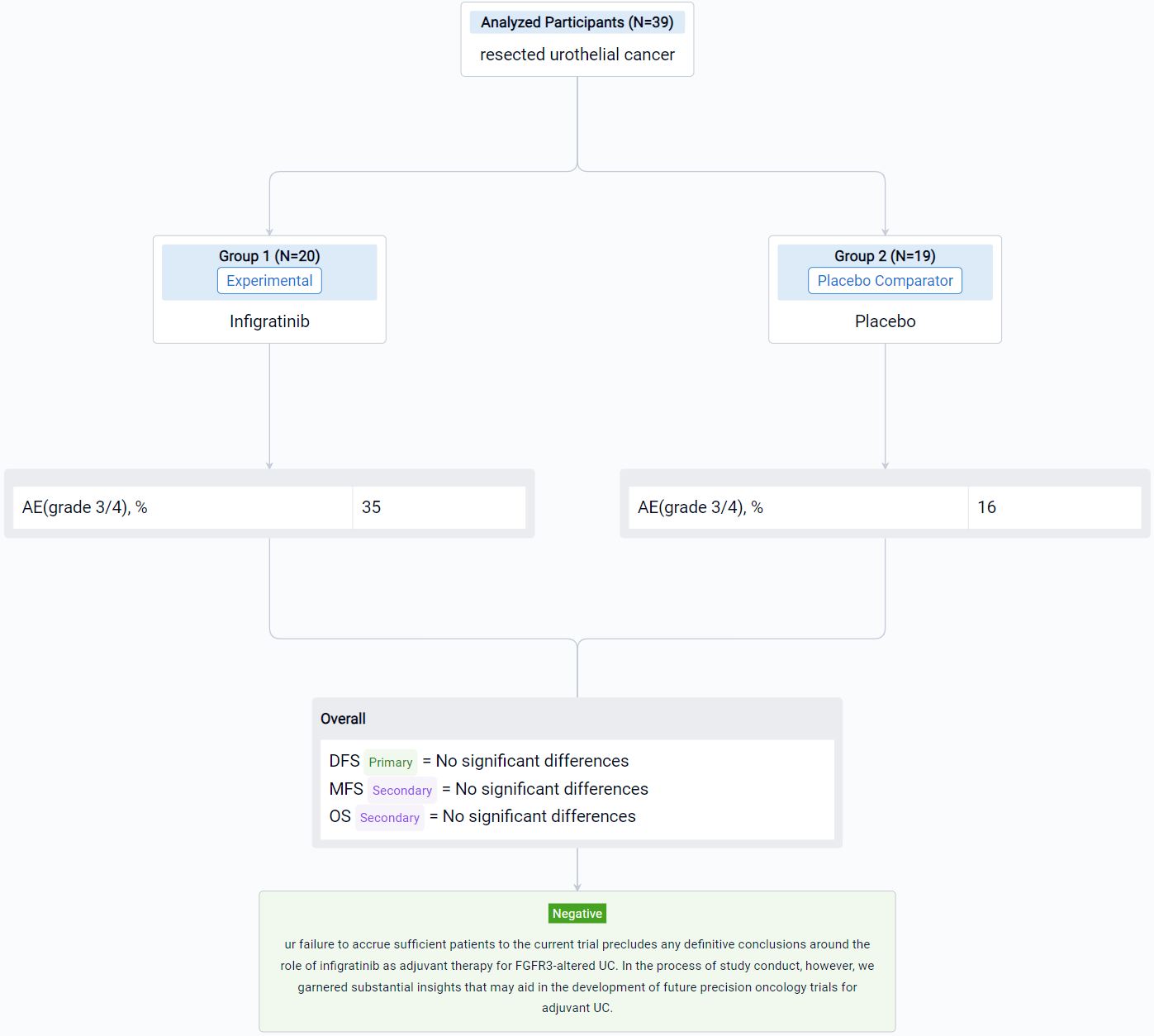
The result showed that despite intensive efforts to enroll approximately 218 patients (with 822 patients consented to molecular pre-screening), only 39 patients were enrolled with 20 and 19 patients randomized to receive infigratinib and placebo, respectively. The frequency of FGFR3 alteration was significantly lower than anticipated, occurring in only 19% of patients overall; mutations were observed in 13% of patients with lower tract UC and 30% of patients with upper tract UC. No significant differences were observed in DFS, MFS or OS, and more frequent grade 3/4 adverse events were noted in the experimental arm (35% versus 16%). No fatal adverse events were observed.
It can be concluded that the authors failure to accrue sufficient patients to the current trial precludes any definitive conclusions around the role of infigratinib as adjuvant therapy for FGFR3-altered UC. In the process of study conduct, however, we garnered substantial insights that may aid in the development of future precision oncology trials for adjuvant UC.
How to Easily View the Clinical Results Using Synapse Database?
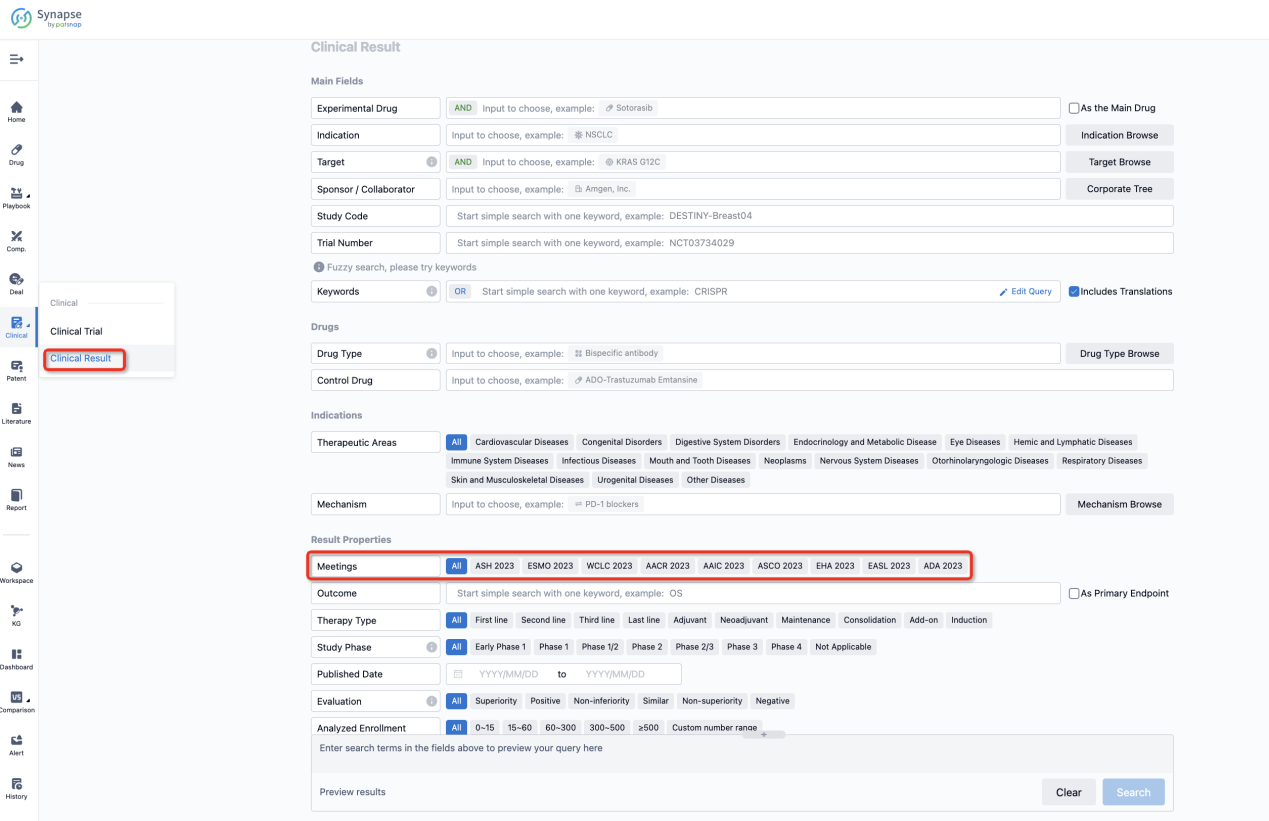
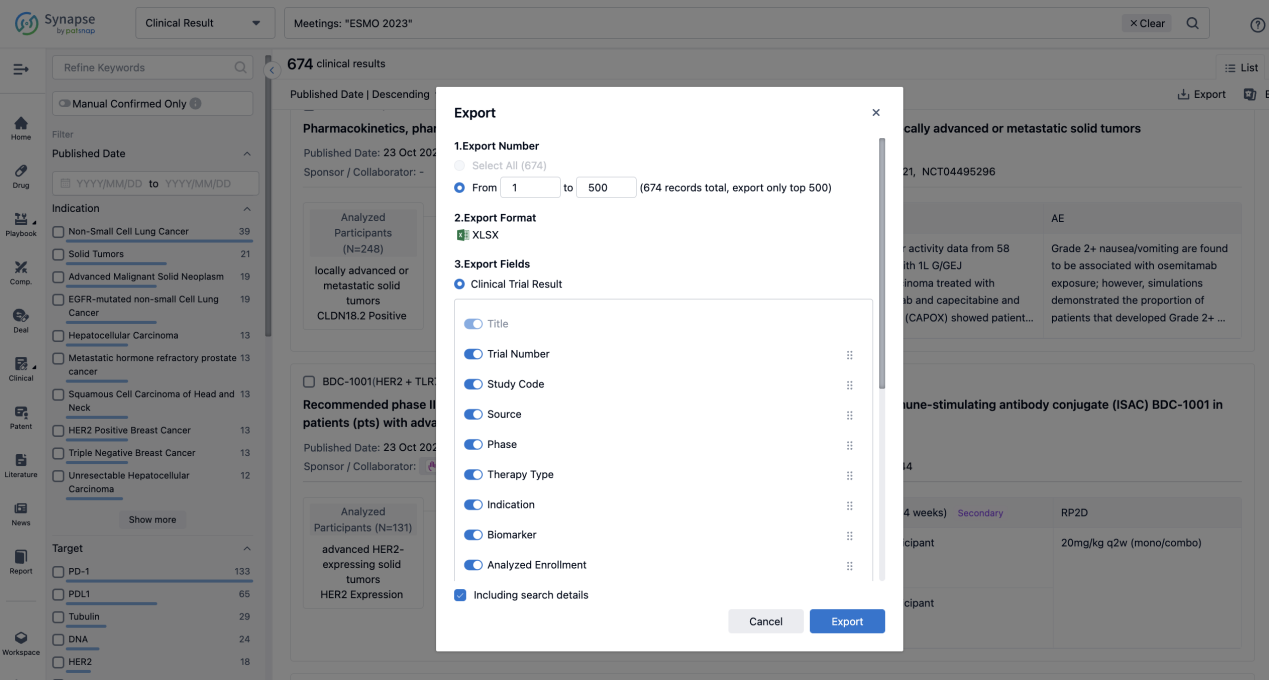
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
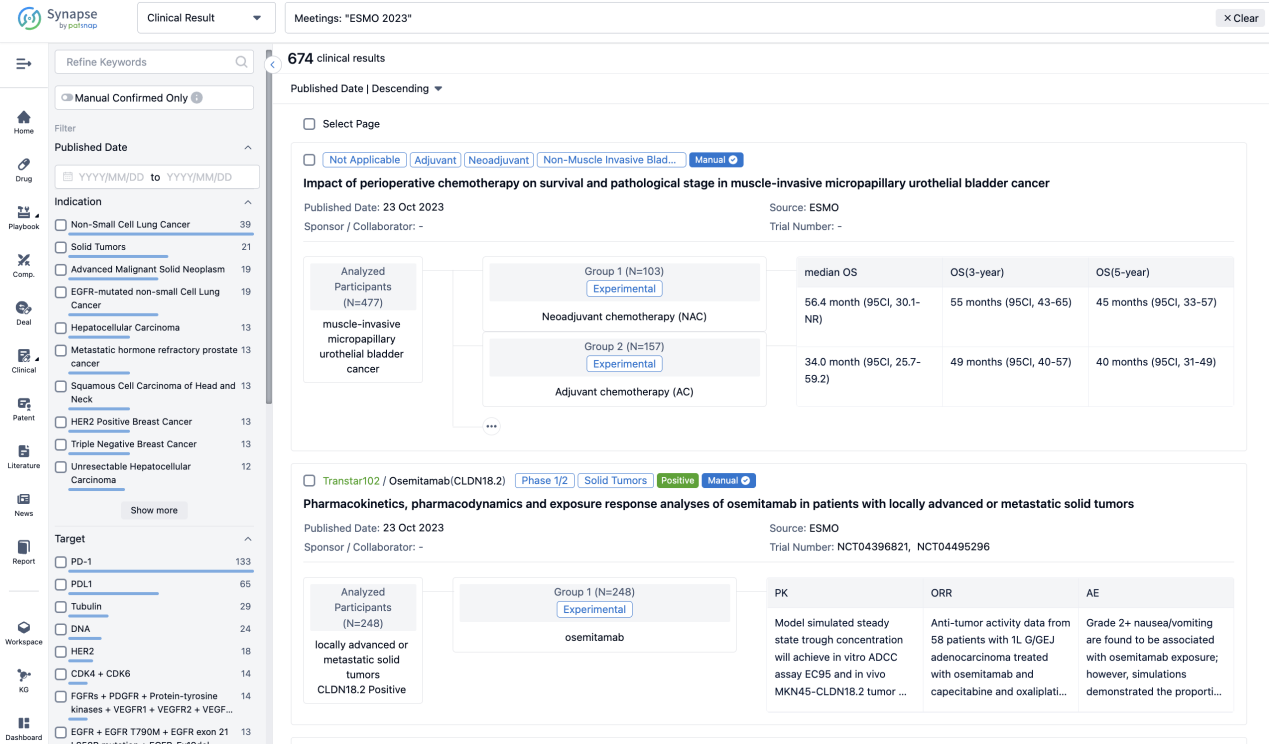
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

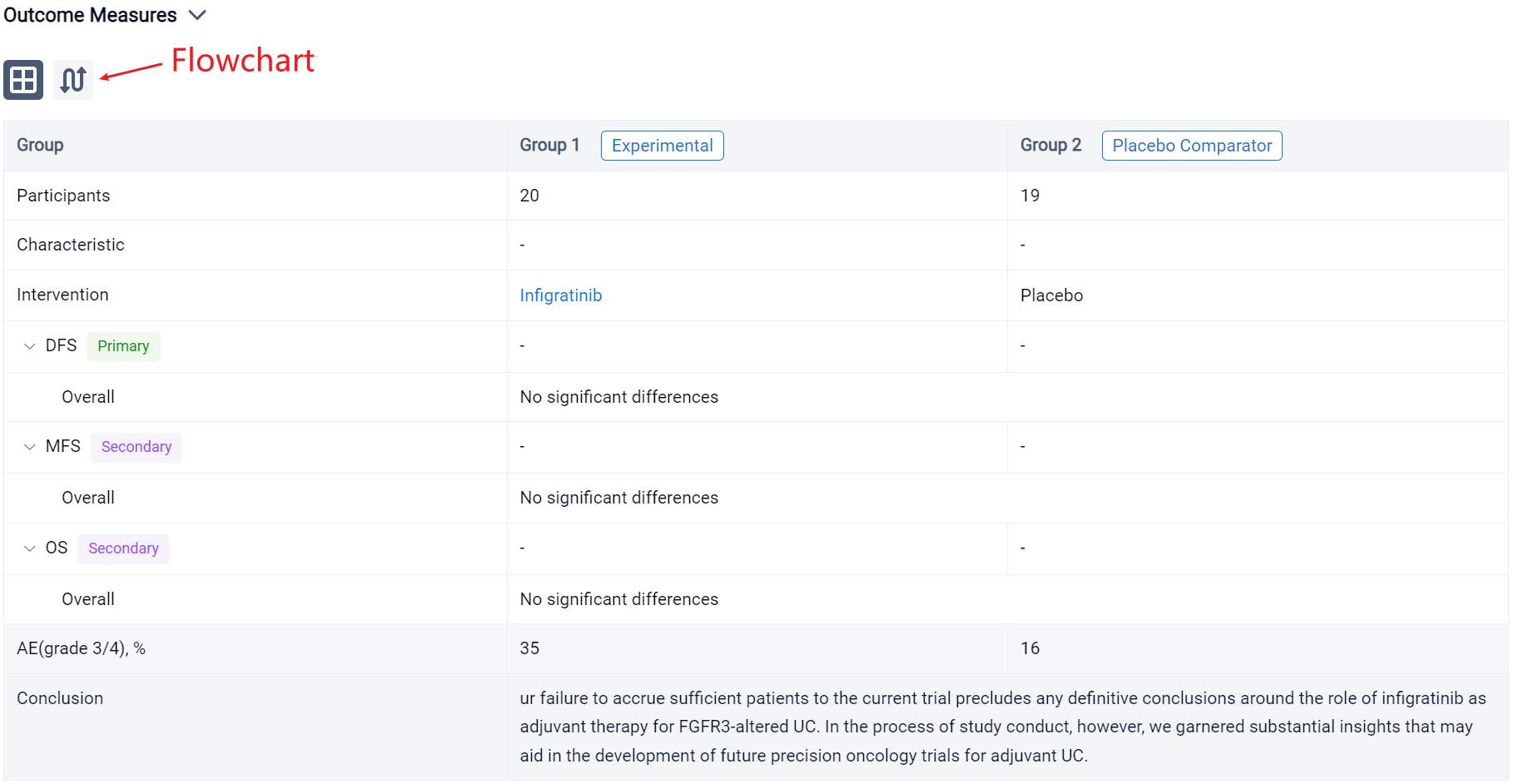
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!