Progress and Potential of Laroprovstat (AZD-0780): A PCSK9 Inhibitor in the Spotlight for Cardiovascular and Metabolic Therapy
Laroprovstat(AZD-0780) is a small molecule drug developed by AstraZeneca PLC, targeting the PCSK9 protein. The drug is primarily focused on treating dyslipidemias, hepatic insufficiency, and cardiovascular diseases, making it relevant in the therapeutic areas of endocrinology and metabolic disease, cardiovascular diseases, and digestive system disorders.
In terms of development, Laroprovstat has reached the Phase 2 stage globally, indicating that it has shown promise in early clinical trials. In China, the drug has received IND (Investigational New Drug) approval, signaling that it can proceed to clinical testing in the country.
As a PCSK9-targeting drug, Laroprovstat offers potential benefits in managing lipid levels and cardiovascular health. Through its action on PCSK9, the drug may help lower cholesterol levels and reduce the risk of cardiovascular events, making it a valuable candidate for addressing conditions such as dyslipidemias and cardiovascular diseases.
Below, we will use the drug Laroprovstat as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
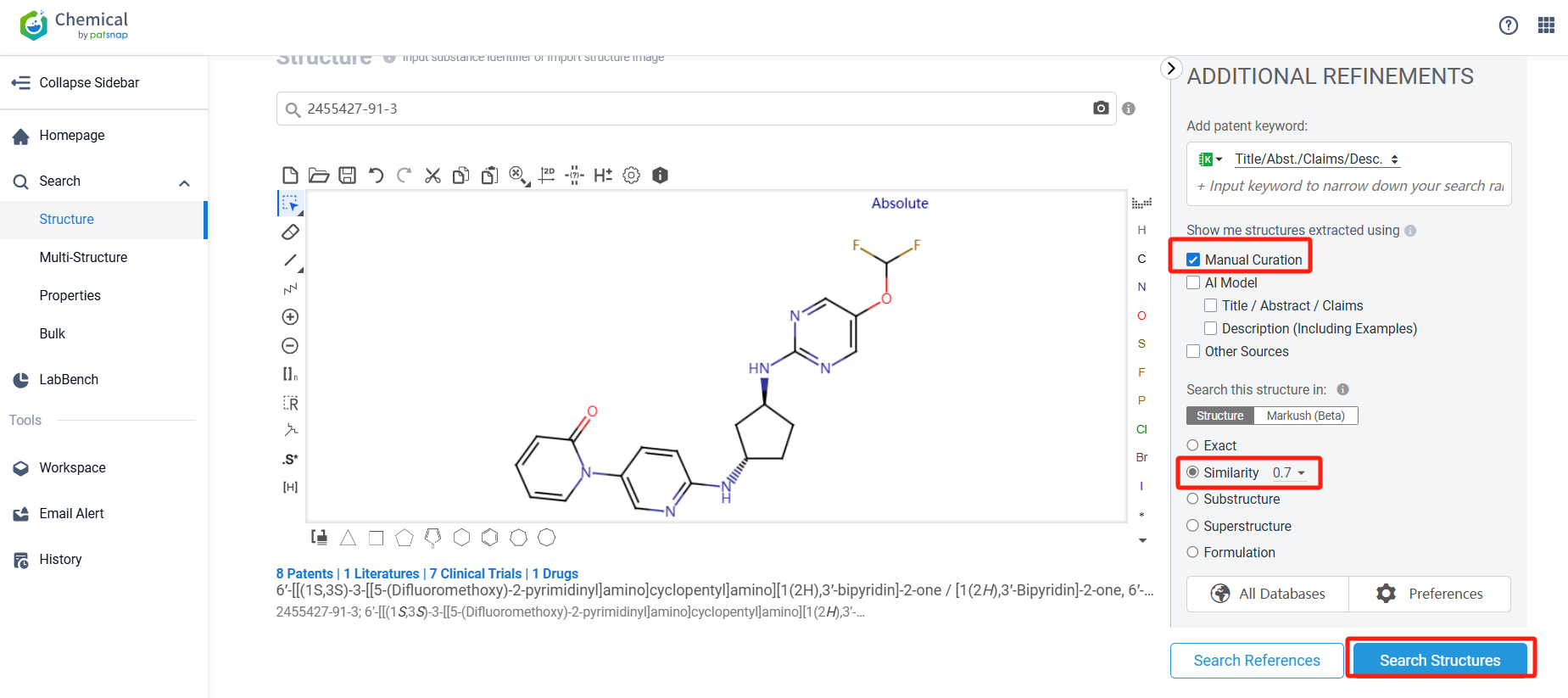
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of Laroprovstat (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, using a similarity search (setting the Tanimoto coefficient to 0.7), check the box for manual curation, click on search structures, and you can find the innovative drug Laroprovstat, as disclosed in the patent application with the publication number WO2020150474A1, first made public on 2023-07-23.
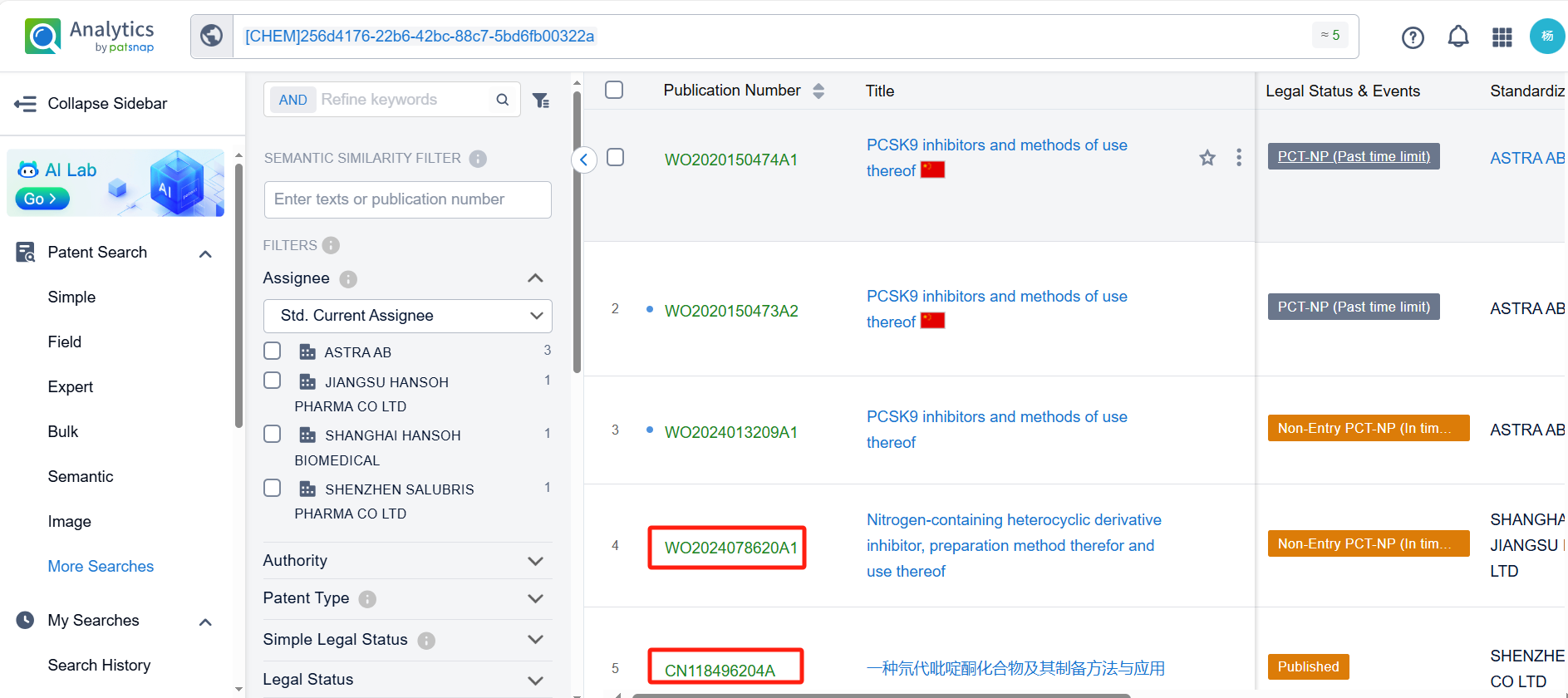
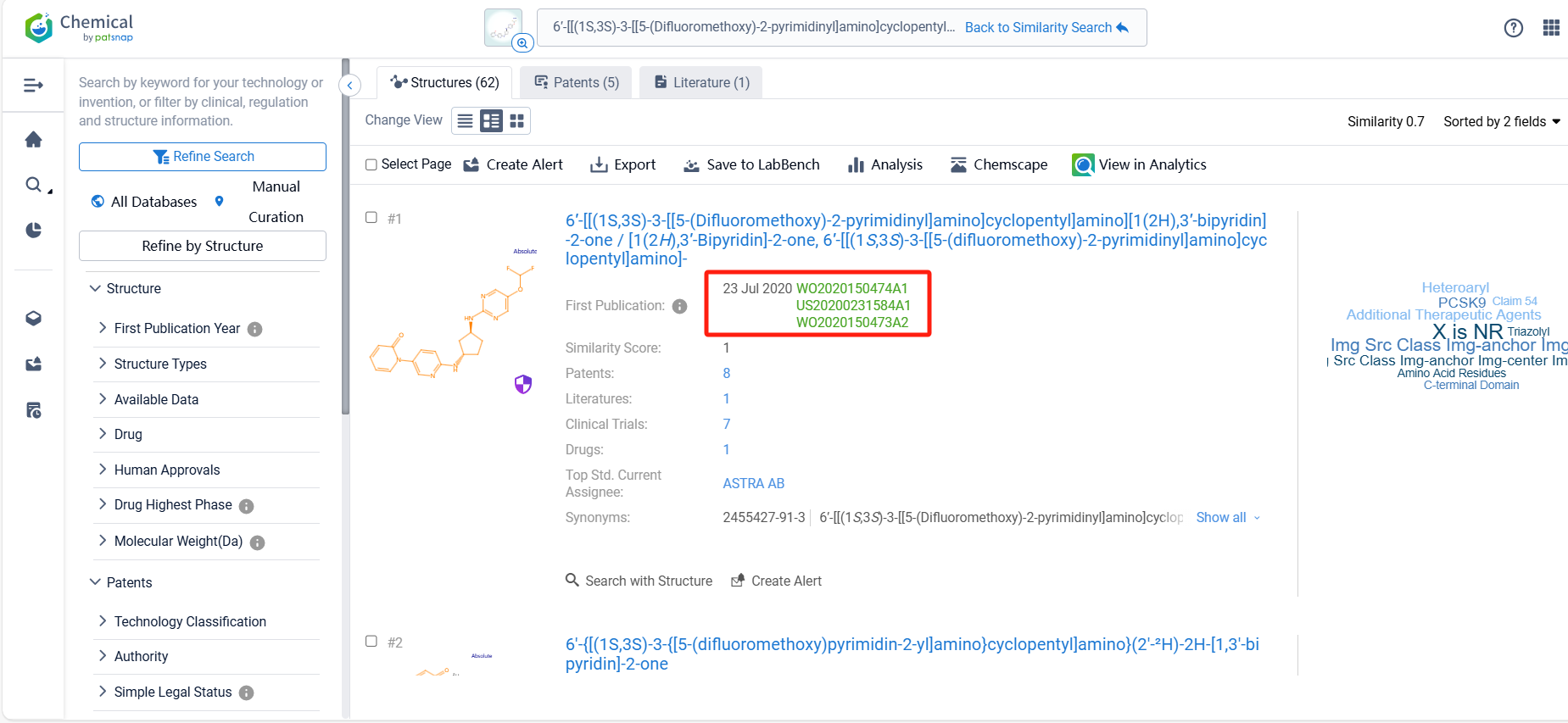 There are 5 patents related to this compound. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
There are 5 patents related to this compound. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
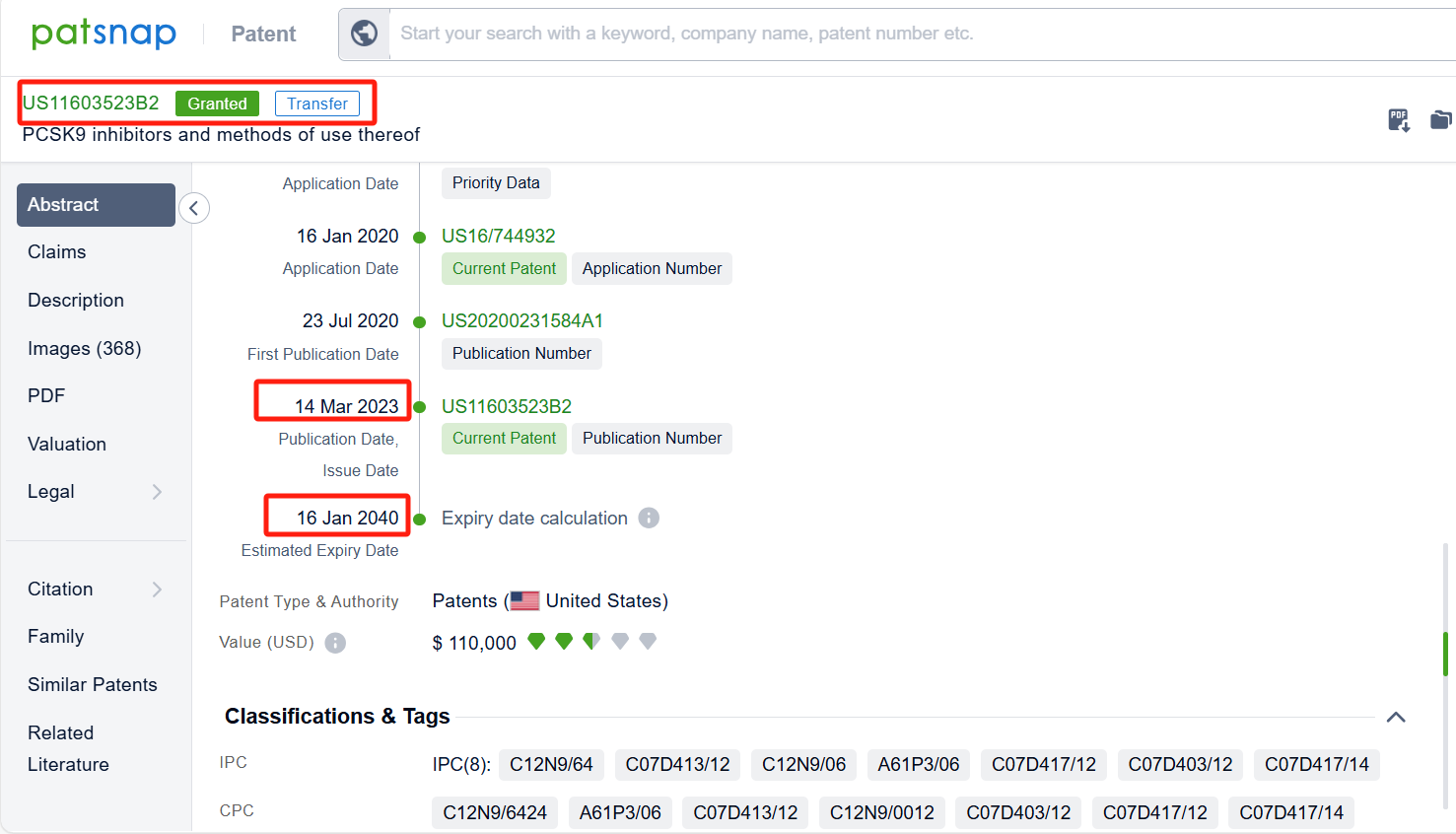
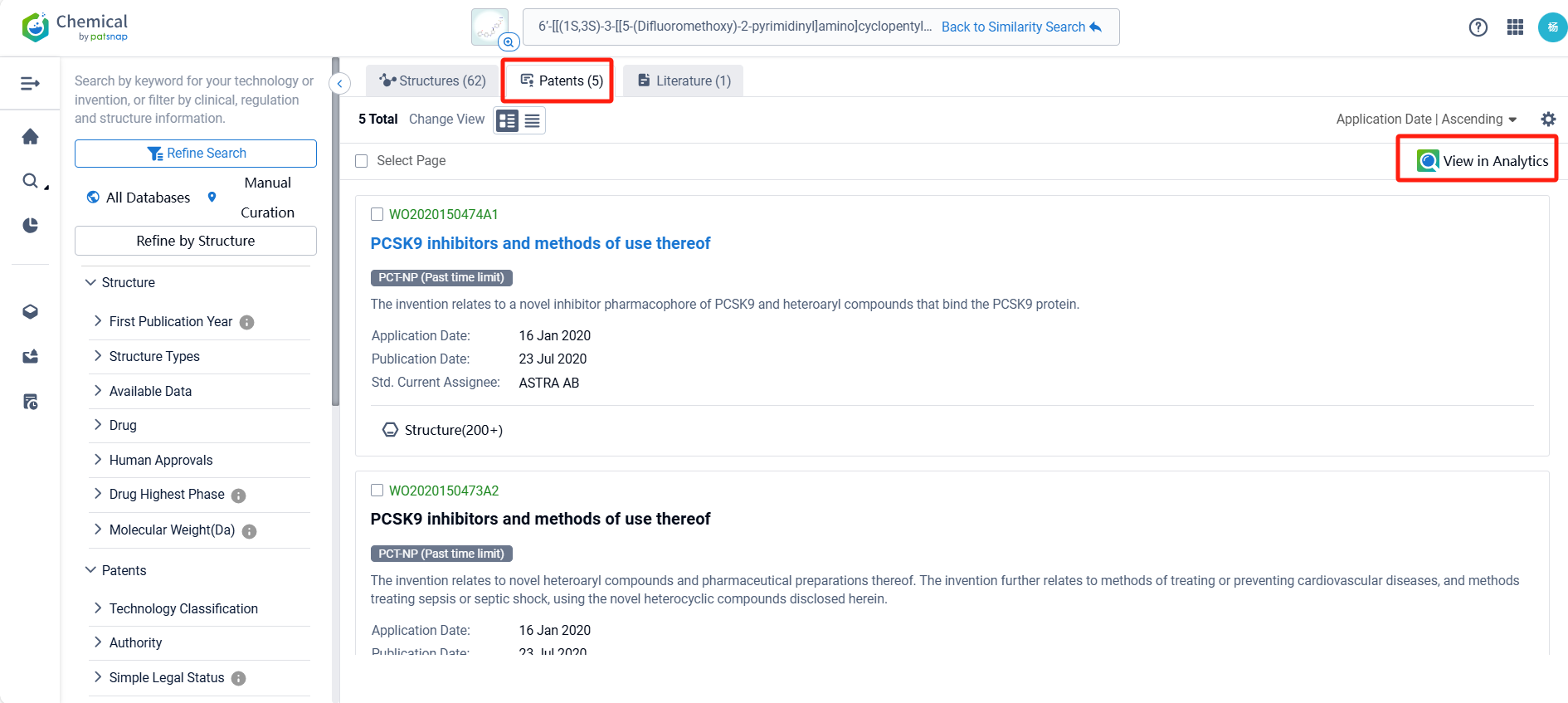 By reviewing the aforementioned patents, we can observe that the core United States patent related to this compound has been granted, with the grant publication number US11603523B2, the grant date being March 14, 2023, and the estimated expiration date January 16, 2040. Its corresponding Chinese patent application CN113412258A has also entered substantive examination.
By reviewing the aforementioned patents, we can observe that the core United States patent related to this compound has been granted, with the grant publication number US11603523B2, the grant date being March 14, 2023, and the estimated expiration date January 16, 2040. Its corresponding Chinese patent application CN113412258A has also entered substantive examination.
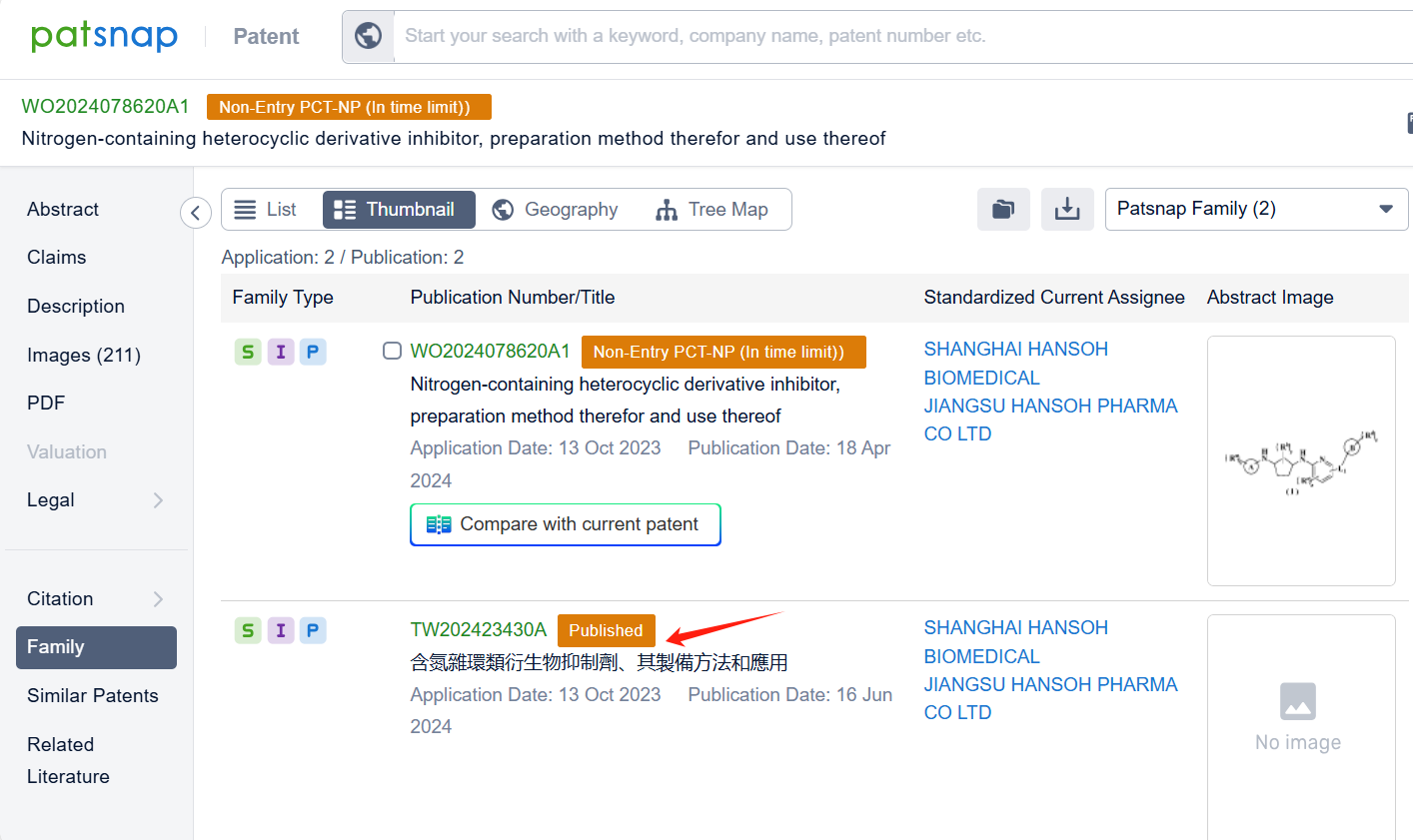
 Among the applicants of the patent, one can find other companies' fast follow patents on AstraZeneca PLC; for example, Shenzhen Salubris Pharma Co., Ltd. has disclosed the isotope derivatives of Laroprovstat in its Chinese patent CN118496204A. The use of isotope derivatives typically extends the half-life, reduces clearance rates, enhances metabolic stability, and improves in vivo activity. Additionally, Shanghai Hansoh Biopharma Co., Ltd. and Jiangsu Hansoh Pharmaceutical Group Co., Ltd. have also published their international patent application WO2024078620A1 on April 18, 2024, and The aforementioned international PCT application has been published in Taiwan, China.
Among the applicants of the patent, one can find other companies' fast follow patents on AstraZeneca PLC; for example, Shenzhen Salubris Pharma Co., Ltd. has disclosed the isotope derivatives of Laroprovstat in its Chinese patent CN118496204A. The use of isotope derivatives typically extends the half-life, reduces clearance rates, enhances metabolic stability, and improves in vivo activity. Additionally, Shanghai Hansoh Biopharma Co., Ltd. and Jiangsu Hansoh Pharmaceutical Group Co., Ltd. have also published their international patent application WO2024078620A1 on April 18, 2024, and The aforementioned international PCT application has been published in Taiwan, China.
 Overall, the development of Laroprovstat represents a significant effort by AstraZeneca PLC in advancing treatments for a range of conditions within the biomedicine field. The drug's progression to Phase 2 globally and IND approval in China reflects a notable level of interest and investment in its potential. As the development of Laroprovstat continues, further clinical data and advancements will provide greater insight into its efficacy and safety in addressing dyslipidemias, hepatic insufficiency, and cardiovascular diseases.
Overall, the development of Laroprovstat represents a significant effort by AstraZeneca PLC in advancing treatments for a range of conditions within the biomedicine field. The drug's progression to Phase 2 globally and IND approval in China reflects a notable level of interest and investment in its potential. As the development of Laroprovstat continues, further clinical data and advancements will provide greater insight into its efficacy and safety in addressing dyslipidemias, hepatic insufficiency, and cardiovascular diseases.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.