Progress in Research on CCR5 Antagonist Drugs
CCR5, or C-C chemokine receptor type 5, is a protein receptor found on the surface of certain immune cells, including T cells and macrophages. It plays a crucial role in the immune response by facilitating the migration of these cells to sites of inflammation or infection. Additionally, CCR5 acts as a co-receptor for the entry of the human immunodeficiency virus (HIV) into target cells, making it an important target for antiviral therapies. Modulating CCR5 activity has shown promise in the treatment of HIV/AIDS and other inflammatory diseases. Understanding the role of CCR5 provides valuable insights for the development of novel pharmaceutical interventions.
CCR5 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 8 Sep 2023, there are a total of 67 CCR5 drugs worldwide, from 81 organizations, covering 67 indications, and conducting 353 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.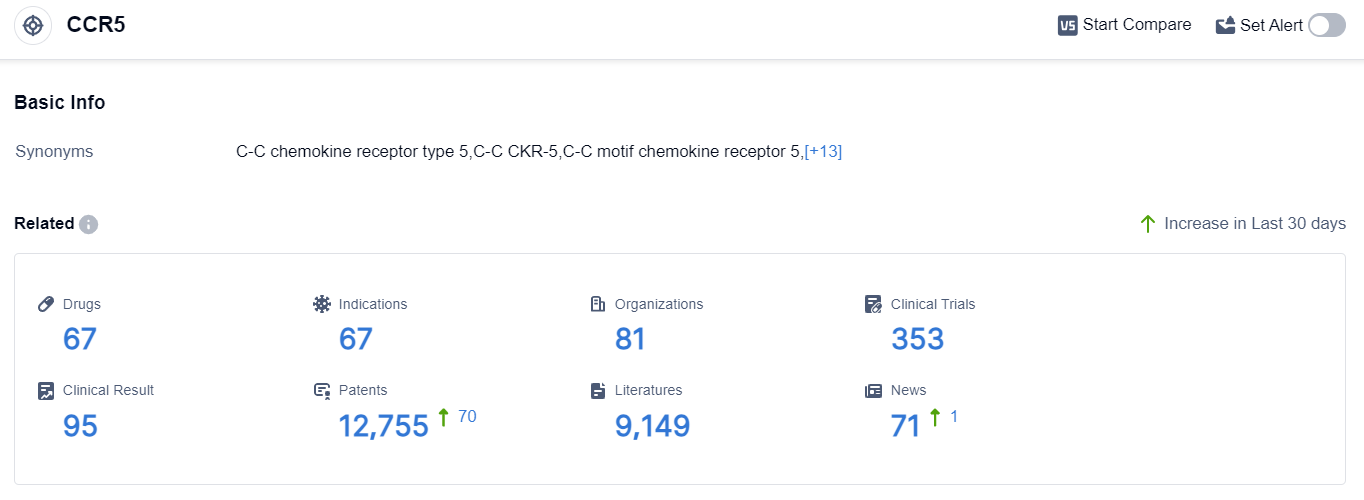
The current competitive landscape of target CCR5 shows that multiple companies are actively involved in the development of drugs. GSK Plc is leading in terms of the highest stage of development, followed by Pfizer Inc., Viatris Inc., and other companies.
The approved drugs under the target CCR5 are indicated for various conditions, including HIV Infections, COVID-19, Nonalcoholic Steatohepatitis, and others. Small molecule drugs and monoclonal antibodies are the most rapidly progressing drug types.
The United States, European Union, Canada, and China are among the countries/locations with significant progress in the development of drugs under the target CCR5. China has shown notable progress with an approved drug and ongoing research in the preclinical stage. The future development of target CCR5 is expected to continue with intense competition and advancements in various drug types and indications.
The first approved CCR5 antagonist: Maraviroc
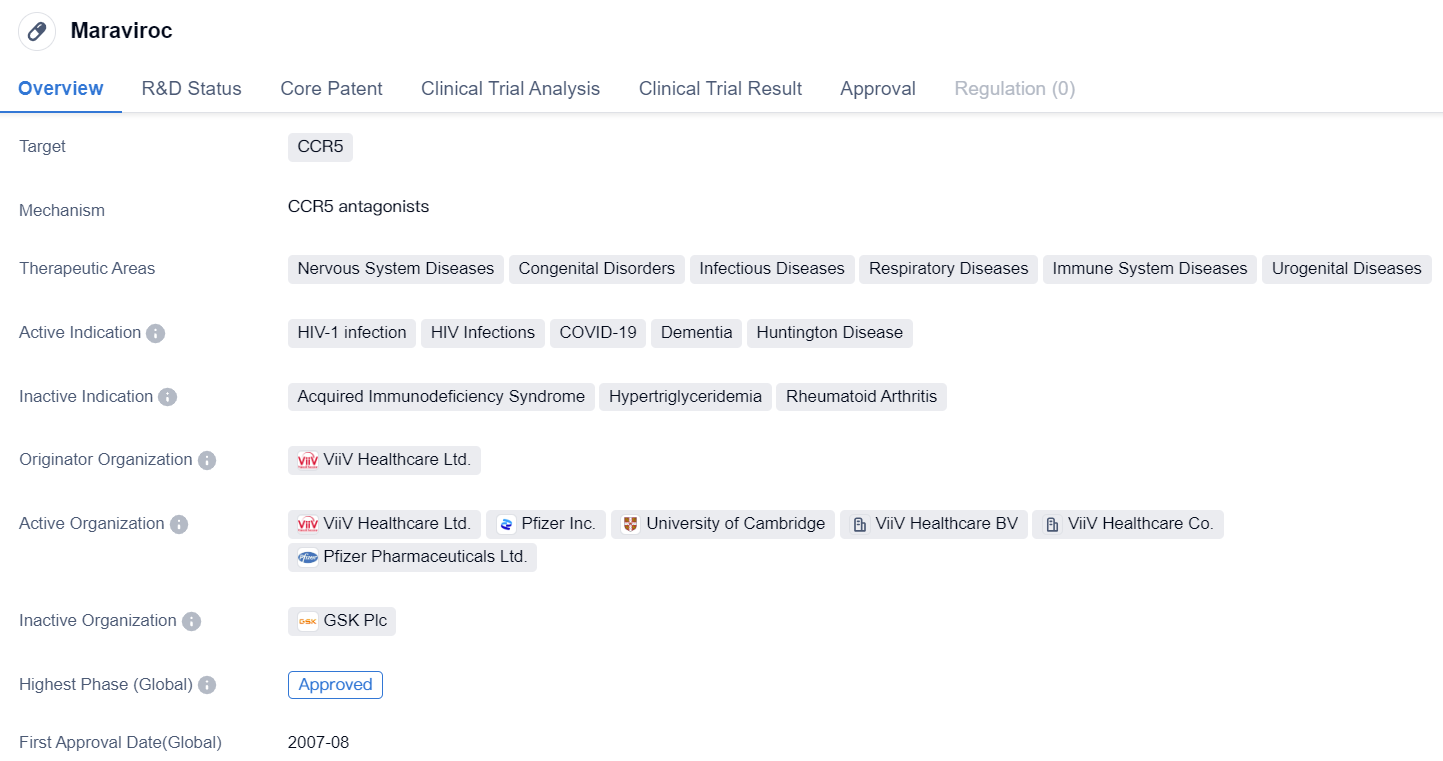
Maraviroc is a small molecule drug that falls under the therapeutic area of biomedicine. It specifically targets CCR5, a protein receptor found on the surface of certain immune cells. This drug has shown potential in treating various diseases related to the nervous system, congenital disorders, infectious diseases, respiratory diseases, immune system diseases, and urogenital diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The active indications for Maraviroc include HIV-1 infection, HIV infections, COVID-19, dementia, and Huntington disease. It is important to note that Maraviroc is not a cure for these conditions but rather a treatment option that can help manage symptoms and improve the quality of life for patients.
ViiV Healthcare Ltd. is the originator organization behind Maraviroc. This pharmaceutical company has played a significant role in the development and commercialization of this drug. Maraviroc has successfully completed the highest phase of clinical trials and has received approval both globally and in China.
The first approval for Maraviroc was granted in the United States in August 2007. This signifies that the drug has met the necessary regulatory requirements and has been deemed safe and effective for use in patients. It is worth mentioning that Maraviroc's approval for COVID-19 indicates its potential in combating the ongoing pandemic caused by the novel coronavirus.
In summary, Maraviroc is a small molecule drug developed by ViiV Healthcare Ltd. It targets CCR5 and has been approved for various therapeutic areas, including nervous system diseases, congenital disorders, infectious diseases, respiratory diseases, immune system diseases, and urogenital diseases. Its active indications include HIV-1 infection, HIV infections, COVID-19, dementia, and Huntington disease. Maraviroc has received global and Chinese approval, with its first approval granted in the United States in 2007. This drug represents a significant advancement in the field of biomedicine and offers potential benefits for patients suffering from these conditions.
CCR5 Antagonist Entering NDA/BLA: Leronlimab
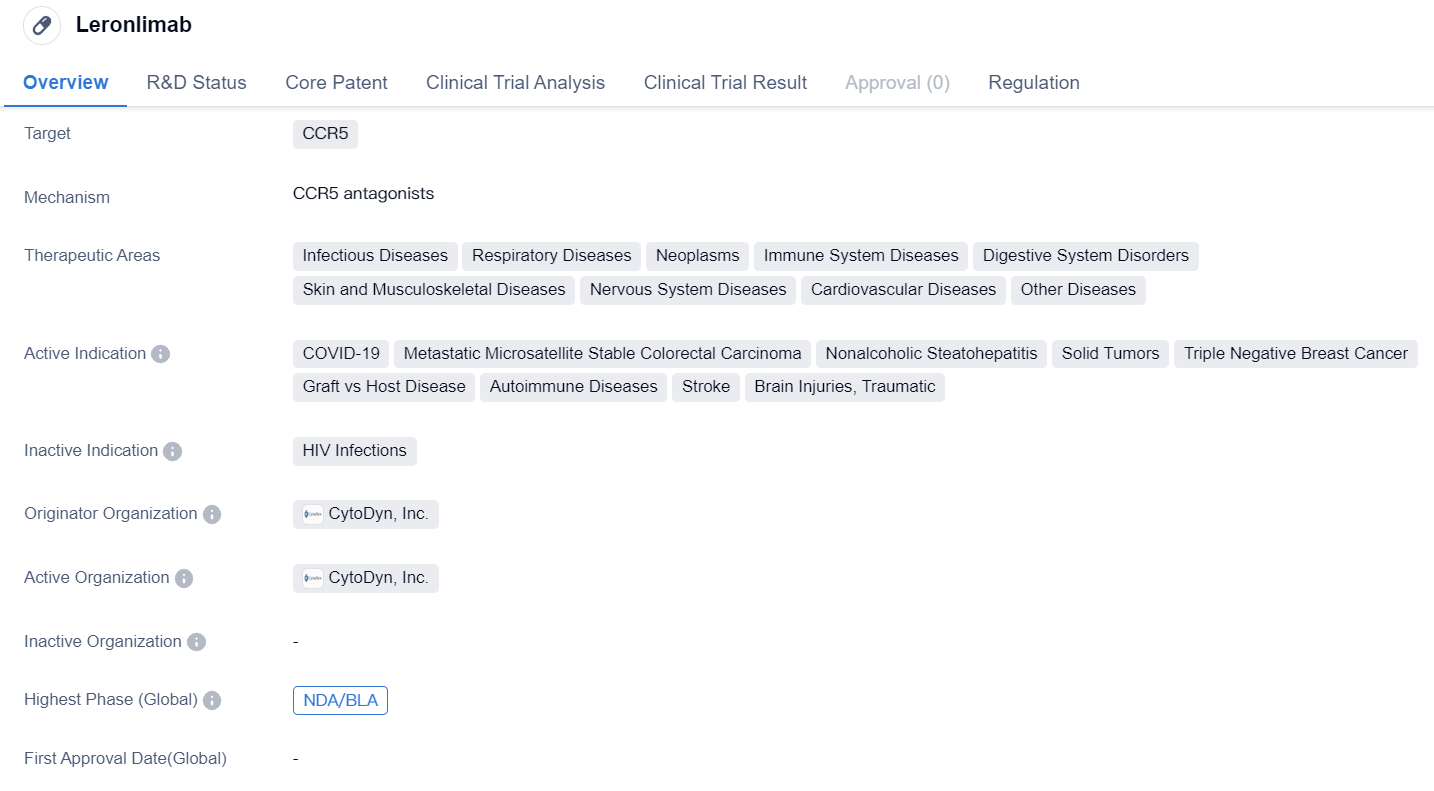
Leronlimab is a monoclonal antibody drug developed by CytoDyn, Inc. It targets CCR5, a receptor involved in various diseases. The drug has shown potential in treating a wide range of therapeutic areas, including infectious diseases, respiratory diseases, neoplasms, immune system diseases, digestive system disorders, skin and musculoskeletal diseases, nervous system diseases, cardiovascular diseases, and other diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In terms of active indications, Leronlimab has demonstrated efficacy in treating COVID-19, metastatic microsatellite stable colorectal carcinoma, nonalcoholic steatohepatitis, solid tumors, triple negative breast cancer, graft vs host disease, autoimmune diseases, stroke, and traumatic brain injuries.
Leronlimab has reached the New Drug Application (NDA) or Biologics License Application (BLA) stage. This indicates that the drug has undergone extensive preclinical and clinical testing and is ready for regulatory approval.
In terms of regulation, Leronlimab has been granted Fast Track designation, which expedites the development and review process for drugs that address unmet medical needs. Additionally, the drug has received Emergency Use Authorization, allowing its use in emergency situations such as the COVID-19 pandemic. Leronlimab has also been designated as an Orphan Drug, indicating its potential to treat rare diseases.
Overall, Leronlimab shows promise as a versatile drug with potential applications in various therapeutic areas. Its targeting of CCR5 and its diverse active indications suggest its potential to address unmet medical needs in multiple disease areas. The drug's advanced stage of development and regulatory designations further support its potential for future commercialization and widespread use.