Progress in the Research and Development of p53 Y220C Drug Targets
The Y220C target mutation is a common oncogenic missense mutation, with a slightly lower mutation frequency compared to R249S and G245S located on the DNA binding surface (it accounts for only 1.4% of p53 missense mutations).
According to World Health Organization statistics, approximately 75,000 cancer cases every year are related to the Y220C mutation. Tyr-220 is located on the surface of the β-sandwich structure, connecting β-strands S7 and S8. Its aromatic ring portion is a component of the hydrophobic core of the β-sandwich structure, but its hydroxyl group can interact with water molecules in the solution. The Y220C mutation leads to a reduction in the thermal stability of the p53 core domain by 4Kcal/mol and results in the mutation of Tyr-220 to Cys-220 between the S3/S4 loop and the S7/S8 loop, forming a hydrophobic cavity on the p53 surface, thereby triggering p53's denaturation and aggregation precipitation.
Moreover, the hydrophobic cavity formed by the Y220C mutation is far from the DNA binding functional domain, thus making this hydrophobic pocket an ideal site for stabilizer design. Stabilizer design based on this hydrophobic cavity does not affect the binding of p53 to DNA or its ability to inhibit cancer.
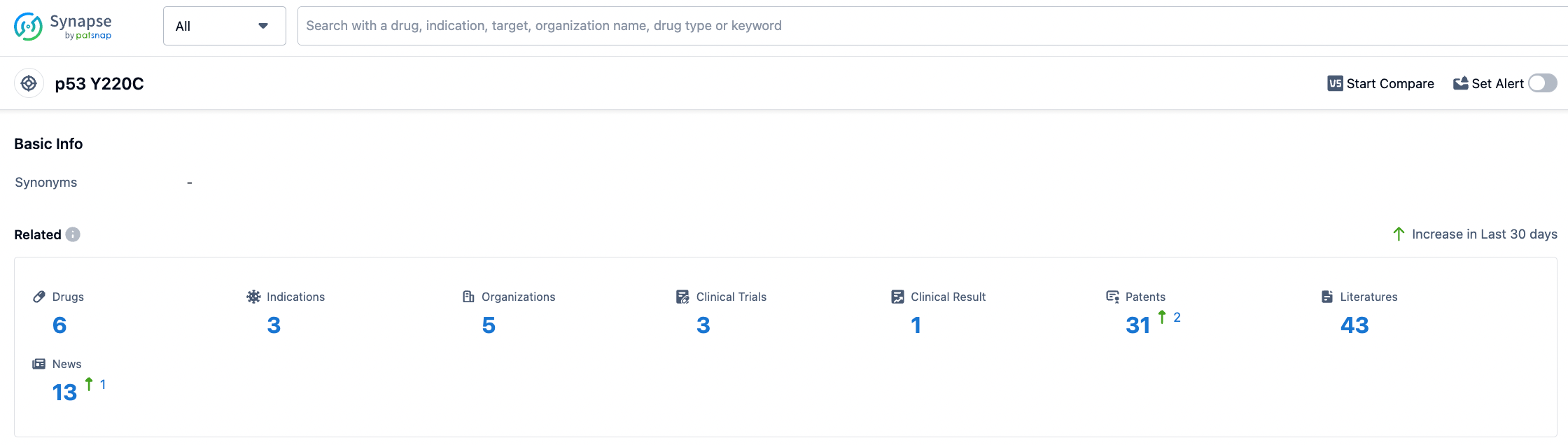
According to data from the Synapse database, there are currently six drugs in the research and development stage targeting p53 Y220C. These drugs cover three different indications, with five organizations developing these drugs. One of these drugs have achieved positive results in clinical trials, providing a solid basis for further development.
In addition, there are already 31 patents and 43 articles related to research on p53 Y220C target drugs, reflecting the scientific activity and knowledge accumulation in this field. With continuous progress in research, we believe that p53 Y220C target drugs will bring new breakthroughs for the treatment of related diseases.
Competitive Landscape
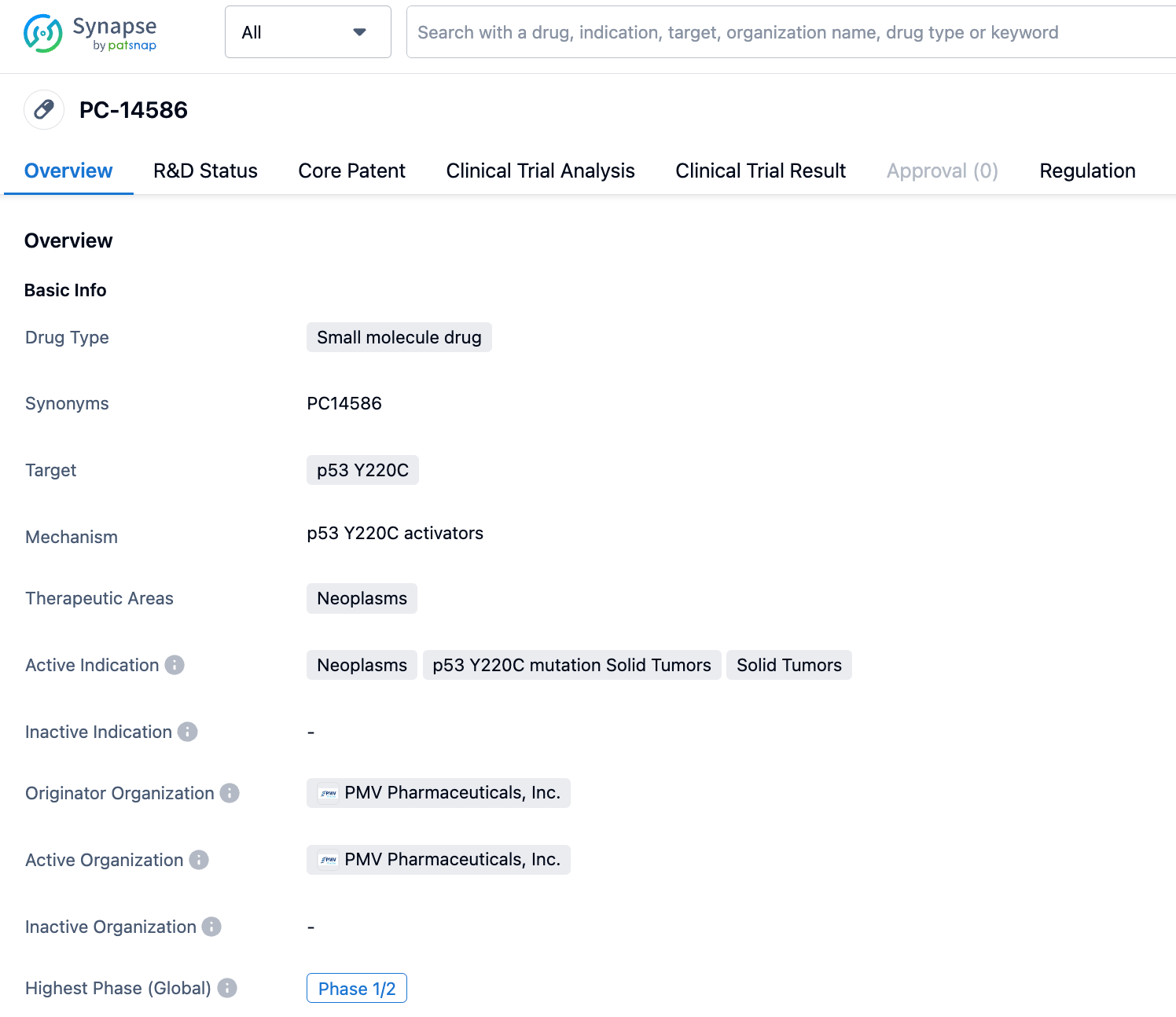
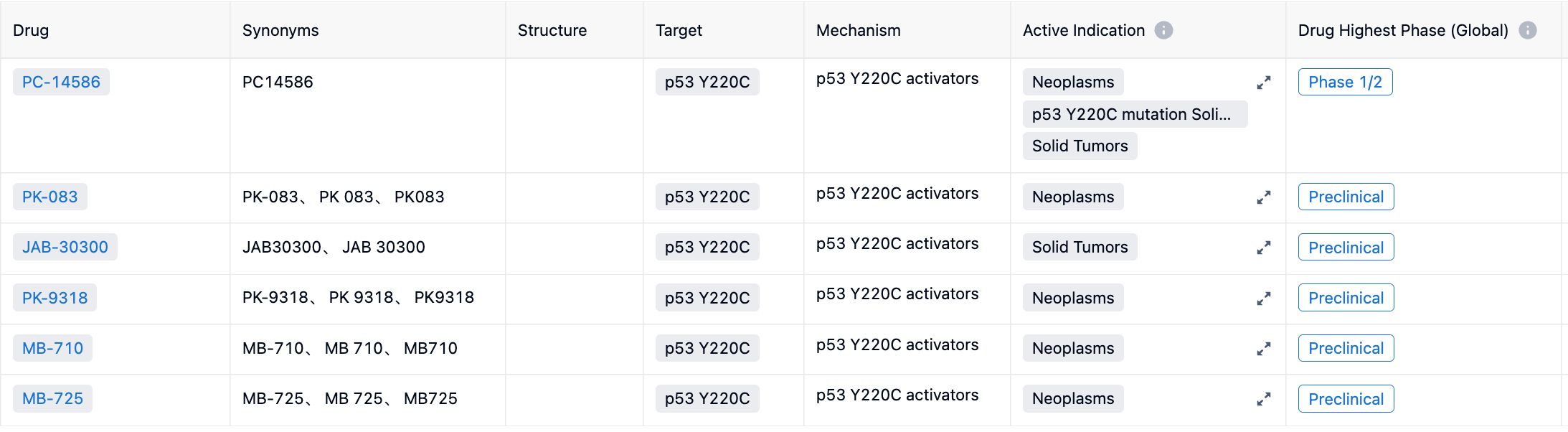
Accessing the Synapse database, you can find 6 drug entries by searching for p53 Y220C drug target. Among them, PC-14586 is in Phase 1/2 clinical trials; MB-710 and MB-725 from MRC Laboratory, JAB-30300 from Jacobio Pharmaceuticals, PK-083 from Cambridge University, and PK-9318 from Cambridge BioMedical are all in preclinical stages.
Key Drug: PC14586
PMVPharma has reported on the small molecule activator PC14586, the structure of which has yet to be disclosed, but which can bind specifically to the Y220C pocket, making it the first Y220C reactivator to enter clinical trials. Preclinical data show that PC14586 can induce tumor regression in xenograft models of Y220C p53 mutant tumors, with an IC50 of ~192-722nM in cell lines derived from Y220C mutant lymphomas and sarcoma tumors. Moreover, PC14586 showed synergistic antitumor activity when combined with anti-PD-1 therapy, as evidenced by the results from the Phase I clinical trial of PC14586.
In terms of safety, the most common treatment-induced adverse events (>15%) include nausea, vomiting, increased AST, increased ALT, anemia, increased creatinine, and fatigue. The maximum tolerable dosage (MTD) is set to be 1500 mg, taken twice daily.
In terms of effectiveness, researchers evaluated according to RECIST v1.1, the ORR of patients with an initial daily dose of 1150 mg or more is 32% (8/25). Among the 8 patients who responded, 6 have confirmed partial response, and 2 have unconfirmed partial response and are awaiting confirmation. The ORR of all dose groups is 24% (8/33). Responses were observed in six different types of tumors, including breast cancer, endometrial cancer, prostate cancer, pancreatic cancer, ovarian cancer, and small cell lung cancer. When the dose is ≥1150mg, the best response of disease stability or partial relief was observed in 19/25 patients (76%).
PMV Pharma has applied for 11 patents around this target, covering compound patents, use patents, P53 mutation detection method patents, co-crystal patents of small molecules and P53 proteins, and more. The earliest patent originated from WO2017143291, which disclosed a class of indole compounds with an acetylene substitution. The latest patent, US2023024905, disclosed the co-crystal of this type of indole compound with an acetylene substitution and P53 protein.
The agonists targeting the mutated p53 Y220C as a novel treatment strategy is expected to treat more than 125,000 cancer patients carrying the p53 Y220C mutation each year, and as a preventative treatment to reduce the cancer risk of Li-Fraumeni syndrome.