AstraZeneca leads the development of small molecule RXFP1 agonists
Relaxin is a pleiotropic hormone that mediates systemic hemodynamics and renal adaptive changes during pregnancy. Relaxin has also been shown to have anti-fibrotic properties and beneficial effects on heart failure, such as acute decompensated heart failure (ADHF). Heart failure has a high incidence and mortality rate. Its characteristics include complex tissue remodeling, including myocardial cell death and interstitial fibrosis increase. Relaxin activates many signal cascade reactions, which are beneficial in the conditions of ischemia reperfusion and heart failure.
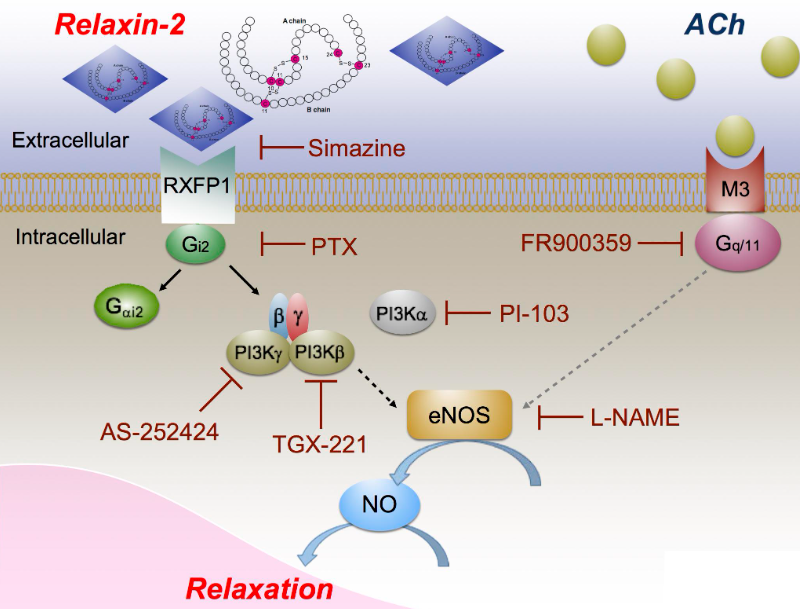
As shown in Figure 1, these signaling pathways include the PI3K pathway and nitric oxide signaling pathway. Relaxin Family Peptide Receptor 1 (RXFP1) is the receptor of relaxin-2, and relaxin-2 is an important regulator of reproduction and cardiovascular physiology. RXFP1 is a well-validated target, a pharmacologically important member of the GPCR family 1c, whose activation by relaxin hormone is associated with hemodynamics, anti-fibrosis, and anti-inflammatory properties. Activation of RXFP1 can reduce vascular resistance and potential lung pressure, thereby increasing cardiac output, enhancing perfusion of important central vascular beds in the kidney, lung, and liver.
Currently, there is only one marketable agonist targeting RXFP1, which is a recombinant natural hormone analogue named serelaxin, developed by Novartis. The agonists in the clinical stage include a Relaxin analogue AZD3427 (Phase II), a small molecule AZD5462 (Phase I), and an mRNA-type agonist mRNA-0184 (Phase I) developed by Moderna Therapeutics, which has been highly recognized for its COVID-19 vaccine. Also, there is a pre-clinical stage peptide agonist CGEN-25010 targeting RXFP1 and RXFP2 (no progress).
The Phase III clinical trial for heart failure treatment with serelaxin failed because it did not meet the primary endpoint, leaving it no choice but to be marketed in Russia. Besides, the half-life of serelaxin is only 10 minutes, therefore, it has to be administrated via intravenous injection. Both the Relaxin analogue AZD3427 and mRNA-0184 also require injection. For the sake of enhancing patients' compliance, the development of small molecule agonists has a broad market.
Targeting RXFP1 with small molecules poses significant challenges due to the lack of direct protein structure information and the poor drugability of the small molecules obtained from early high-throughput screenings. In 2013, the National Institutes of Health in the United States screened over 350,000 compounds through high-throughput methods and discovered the compound ML290. Both AstraZeneca (AZ) and Bristol-Myers Squibb (BMS) optimized based on ML290. Eventually, in June 2022, AZD5462 became the first orally taken small molecule RXFP1 agonist entering the clinical stage.
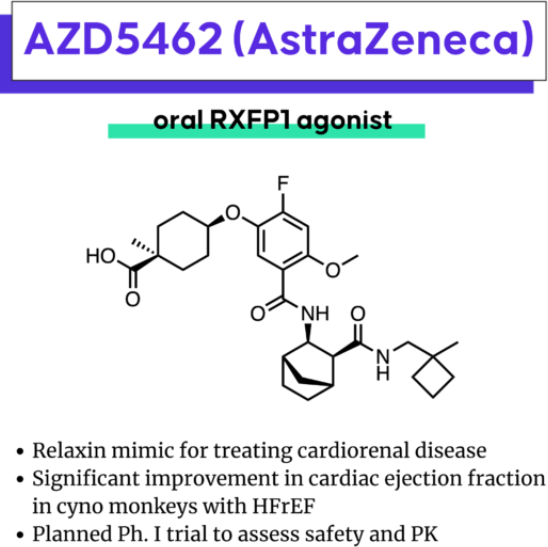
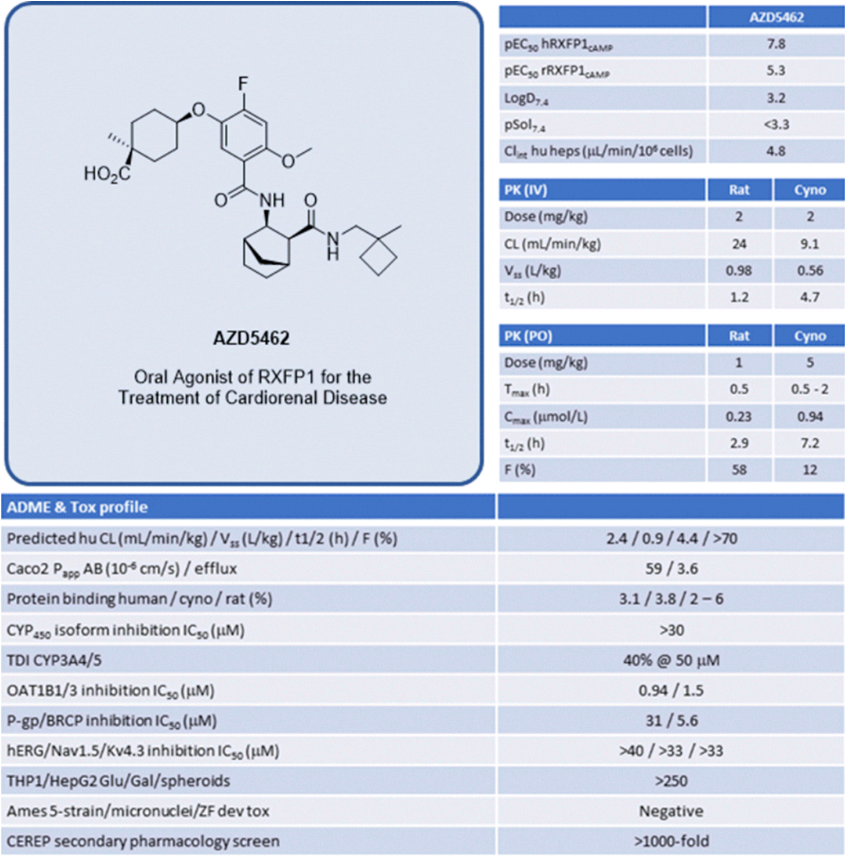
The biennial EFMC-ISMC is a significant conference in the field of medicinal chemistry and drug discovery. At the EFMC-ISMC conference in September 2022, AstraZeneca disclosed the structural information of AZD5462. As shown in Figure 2, the structure of AZD5462 contains an interesting cyclohexane ring reduced to an ethyl group and is connected to a cis-cyclohexanecarboxylic acid fragment. AZD5462 is currently in Phase I clinical trials, where its safety, tolerability, and pharmacokinetics are being evaluated in Japanese healthy volunteers with both single and multiple escalating doses. Preclinical studies on monkey models with heart failure reduced ejection fraction (HFrEF) have shown that after 8 weeks of administration, cardiac systolic function was improved, without any significant increase in mean arterial blood pressure or heart rate. AZD5462 exhibits high selectivity for RXFP1, it shows no activity in the seven closest GPCRs (greater than 10µM), and does not interact with targets in the CEREP Safety panel.
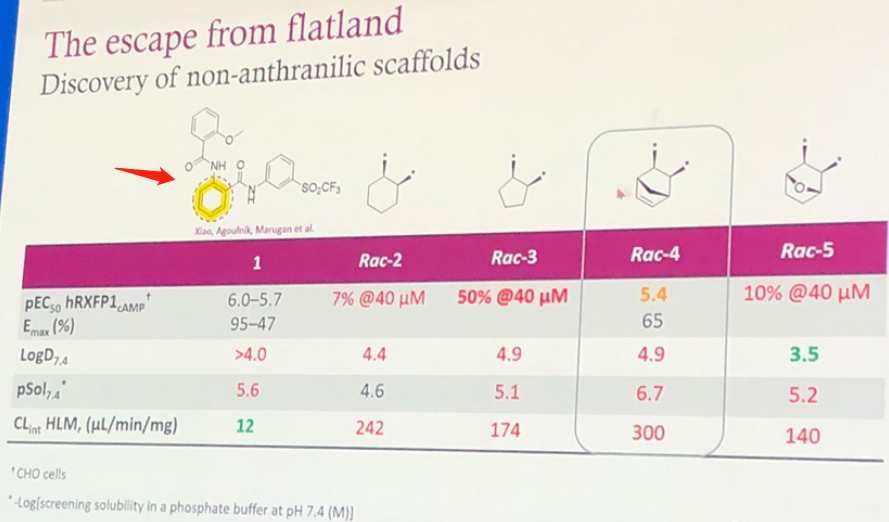
Based on the SAR information disclosed at the conference, it's simple to speculate on the discovery process of AZD5462, which is a case of traditional medicinal chemistry modification. The structure of ML290 is shown by the arrow in Figure 3, and its high LogD7.4 is likely to present toxicity risks. The benzene ring of the para-Aminobenzoic fragment was initially replaced with a non-aromatic fragment, such as in Rac-4 and Rac-5, but both experienced a decrease in activity levels and an increased rate of clearance.
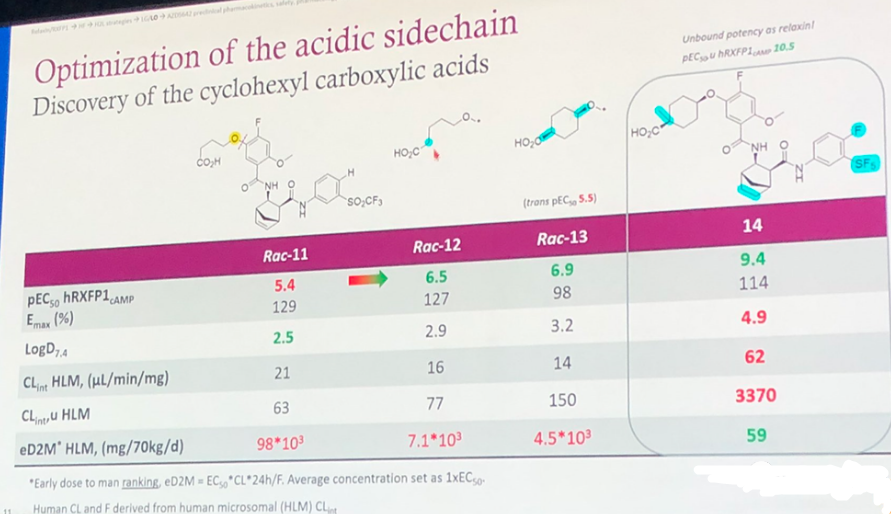
As shown in Figure 4, the key modification involves the incorporation of a carboxyl group. This may have been done to reduce the LogD7.4 or improve the drug's tissue distribution. Indeed, from the subsequently provided Vss, it can be inferred that the volume of distribution was reduced. A comparison between Rac-12 and Rac-13 indicates that having 4 carbon atoms in between the carboxyl group and the oxygen atom is highly beneficial for increasing activity, with improvements of over ten times compared to Rac-11. Further attaching a cyclohexane carboxylic group and saturating the double bond in the non-aromatic ring of Rac-11 resulted in compound 14, which displayed a 10,000-fold increase in activity compared to Rac-11. However, the LogD7.4 of compound 14 remains high at 4.9.
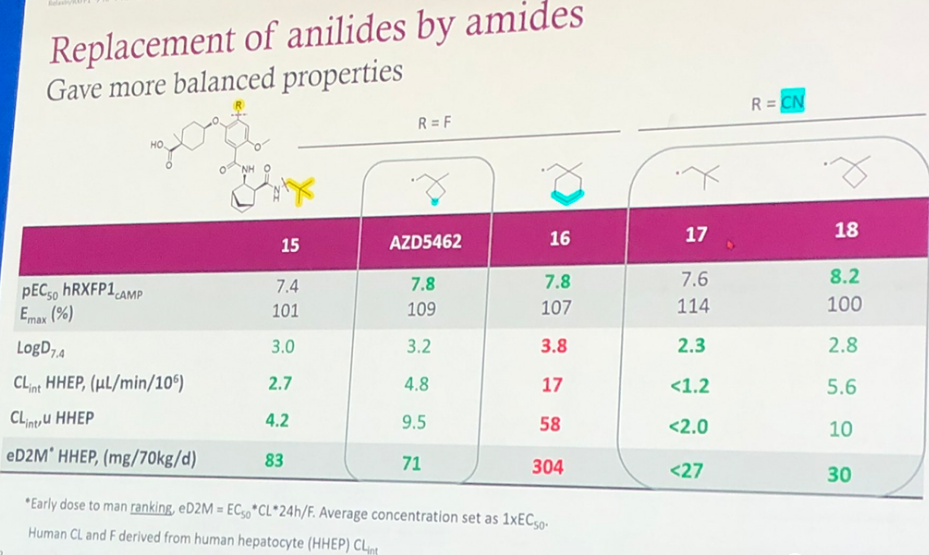
Further, replacing the benzene ring on the far right with non-aromatic fragments to reduce lipophilicity, decrease LogD7.4, and introducing tert-butyl, cyclobutyl, cyclohexyl, etc., produced the compounds 15, AZD5462, 16, 17, 18 as shown in Figure 5. Although the activity decreased, other properties were balanced. For example, LogD7.4 is within a reasonable range and the clearance rate will not be too fast. The predicted human administration dose is also within 100mg/70kg/d (except for compound 16, where the predicted dose reaches 304mg/70kg/d). However, in compounds 17 and 18, the R fragment has been changed from F to CN, reducing the predicted human dose but was not selected. The author speculates this might have been based on considerations of safety.
As shown in Figure 6, the various data for AZD5462 is reorganized. Due to the fact that the indications targeted by the RXFP1 agonist are chronic diseases, safety is undoubtedly the most important. The properties of AZD5462 are balanced, and in the evaluation of the most critical safety parameters, there is no risk of CYP450 enzyme inhibition, no TDI risk (CPY3A4/5), no hERG toxicity risk, and no Ames toxicity risk. In the Caco-2 test, although the efflux reached 3.6 and there was a certain degree of excretion, good absorption compensated for the risk of excretion. Although there is a certain degree of inhibition on the transporters OATP1B1 and OATP1B3, the impact of the drug concentration in the blood under specific dosing should still be considered. In addition, the LogD7.4 of AZD5462 was successfully lowered to 3.2 due to the de-aromatization of two aromatic rings and the introduction of carboxylic acid fragments. The target selectivity in the CEREP Safety panel is also more than 1000 times. Vss is within a reasonable range and will not lead to excessively widespread tissue distribution and affect the efficacy of the drug. The bioavailability in rats reached 58%, and although the bioavailability in crab-eating monkeys was only 12%, the predicted bioavailability in human bodies reached 70% based on the differences in the metabolic stability of AZD5462 in hepatocytes of different species.
Taking into account the above benefit-risk ratio brought about, AZD5462 finally entered phase I clinical trials to evaluate its potential for treating cardiovascular disorders.
In Patsnap Patent Analytics Database, RXFP1 has been searched, where it is mainly Astrazeneca and Bristol-Myers Squibb that are laying out the targeting point RXFP1. There are no patents disclosed by domestic companies following it yet.
Astrazeneca has applied for a patent WO2022122773A1, which contains 624 embodiments, including AZD5462.
Bristol-Myers Squibb disclosed four patents related to RXFP1 agonists on May 4, 2023. The first one is WO2023077041A1, which only has 19 embodiments. The second one is WO2023077040A1, featuring 377 embodiments, with a small part of the compounds achieving nanomolar level. The third patent, WO2023077070A1, includes 188 embodiments, with the majority of the compounds achieving nanomolar activity. Structurally, it is further optimized based on the second patent structure. The fourth one is WO2023076626A1, with 976 embodiments. The structure extends the third one, just replacing the pyridine with a phenyl ring. Activities are mostly at the nanomolar level.
It has been a decade since the structural reporting of ML290 by the National Institutes of Health in the United States. When Astrazeneca and Bristol-Myers Squibb entered the field of small molecule RXFP1 agonists, they took ML290 as the lead compound and optimized its medicinal properties according to the traditional medicinal chemical modification concepts. In 2022, the CryoEM structure of the active state RFXP1 was published on bioRxiv, which will stimulate the discovery of more new structural types of RXFP1 small molecule agonists. As AZD5462 enters clinical evaluation for safety and efficacy, the passion for research and development in this field has been reignited. We look forward to more positive results and hope that this will bring good news for patients with cardiovascular diseases.

Reference
1.Ram W. Sabnis. Novel RXFP1Modulators for Treating Heart Failure. ACS Med. Chem. Lett. 2022, 13, 1213−1214.
2.Maik Gollasch et al; RXFP1 Receptor Activation by Relaxin-2 Induces Vascular Relaxation in Mice via a Gαi2-Protein/PI3Kß/γ/Nitric Oxide-Coupled Pathway. doi: 10.3389/fphys.2018.01234.
3.https://drughunter.com/first-disclosures-from-esmc-ifmc-nice-2022/.
4.Pamela Brown et al; Recent trends in medicinal chemistry and enabling technologies. Highlights from the Society for Medicines Research Conference. Drugs of the Future 2023, 48(3): 211-219.



