Tharimmune's Oral Mucosal Film Medication TH104 Targeting Opioid Receptors
In January, Tharimmune's official website announced plans to initiate a Phase 1 clinical trial to evaluate the safety/tolerability and absolute bioavailability of TH104, with preliminary data expected in the second quarter of 2024.
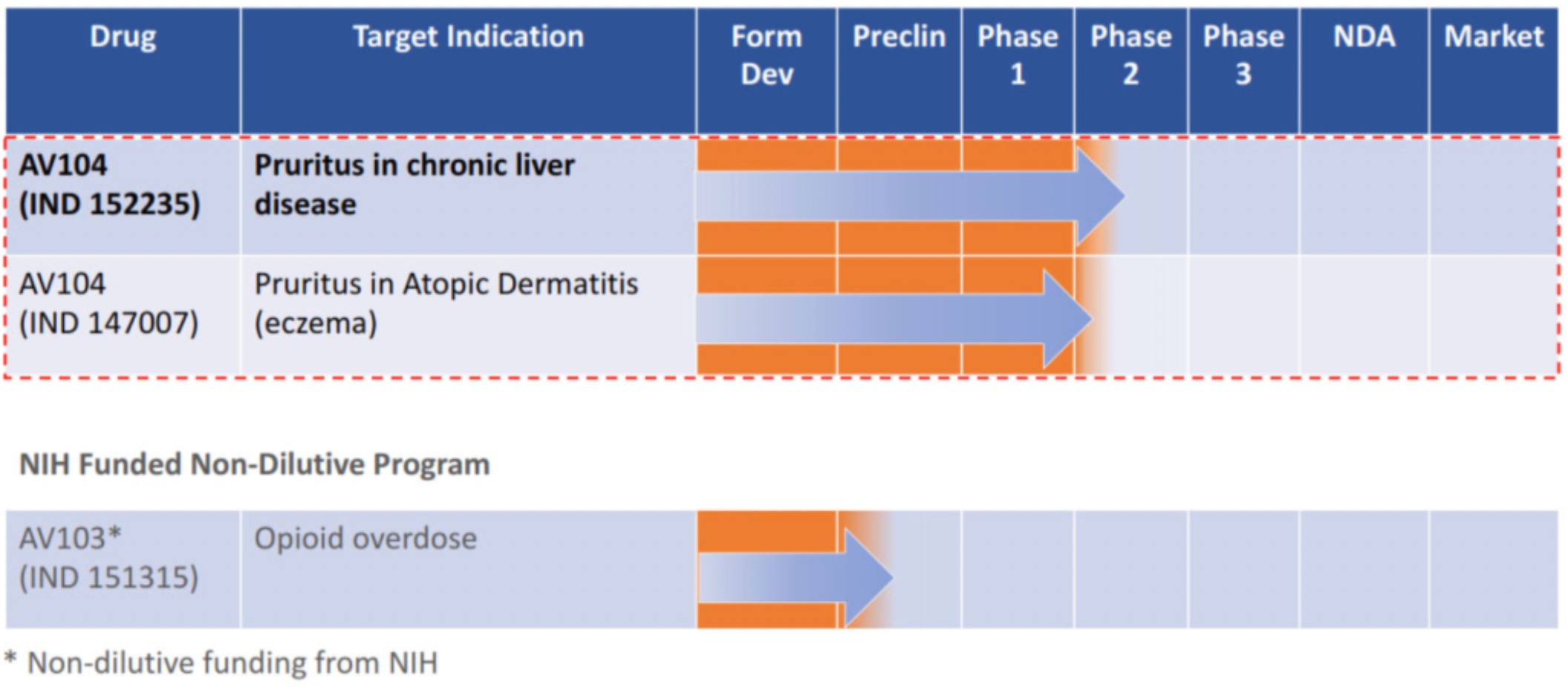
Tharimmune is a clinical-stage biotechnology company developing a pipeline of drug candidates for the treatment of rare, inflammatory, and oncological diseases. According to the latest information released by the company, at the end of 2023, Tharimmune completed the signing of a global exclusive licensing agreement with Avior to develop, market, and commercialize the clinical-stage drug AV104 (to be renamed TH104, hereinafter referred to as TH104).
AV104 is an oral film product of the opioid receptor antagonist Nalmefene, originally developed by Avior. Currently, it is in Phase 2 clinical research for the treatment of pruritus. It is known that AV104 can inhibit chronic, debilitating pruritic diseases or "uncontrollable itching."
According to information provided on Avior Corporation's official website, Speedit is the company's patented oral film with nano/microparticles of medication positioned on the surface of the film. It can easily adhere to the inner cheek within the mouth and is biodegradable within a few minutes.

Tharimmune Corporation intends to first seek approval for TH104 in the orphan disease sector, intended for the treatment of moderate to severe cholestatic pruritus in patients with primary biliary cholangitis (PBC).
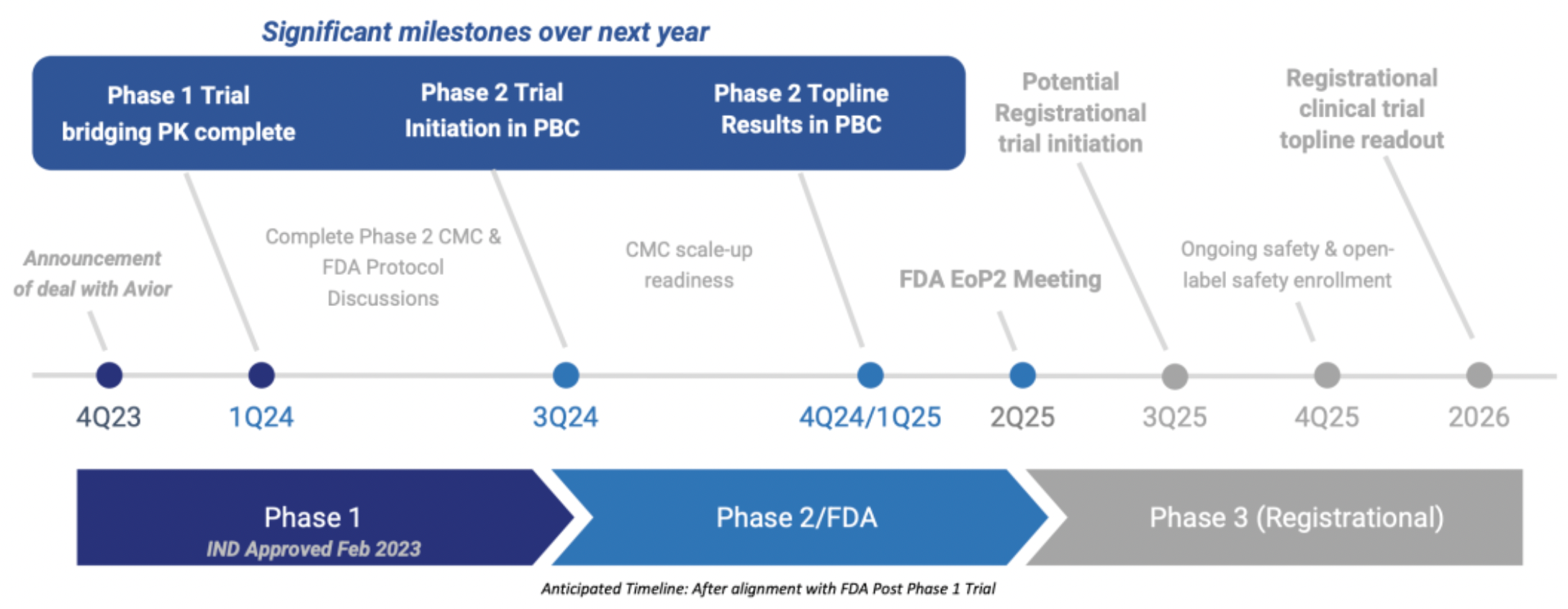
Reportedly, the advantages of TH104 include compatibility with once-daily dosing pharmacokinetics (PK), rapid onset of action (10 minutes), high bioavailability (>70%), and the ability to bypass the liver (no first-pass effect). The medication can be absorbed and distributed to the skin more quickly, which is particularly important in cases where liver damage leads to disordered drug metabolism.
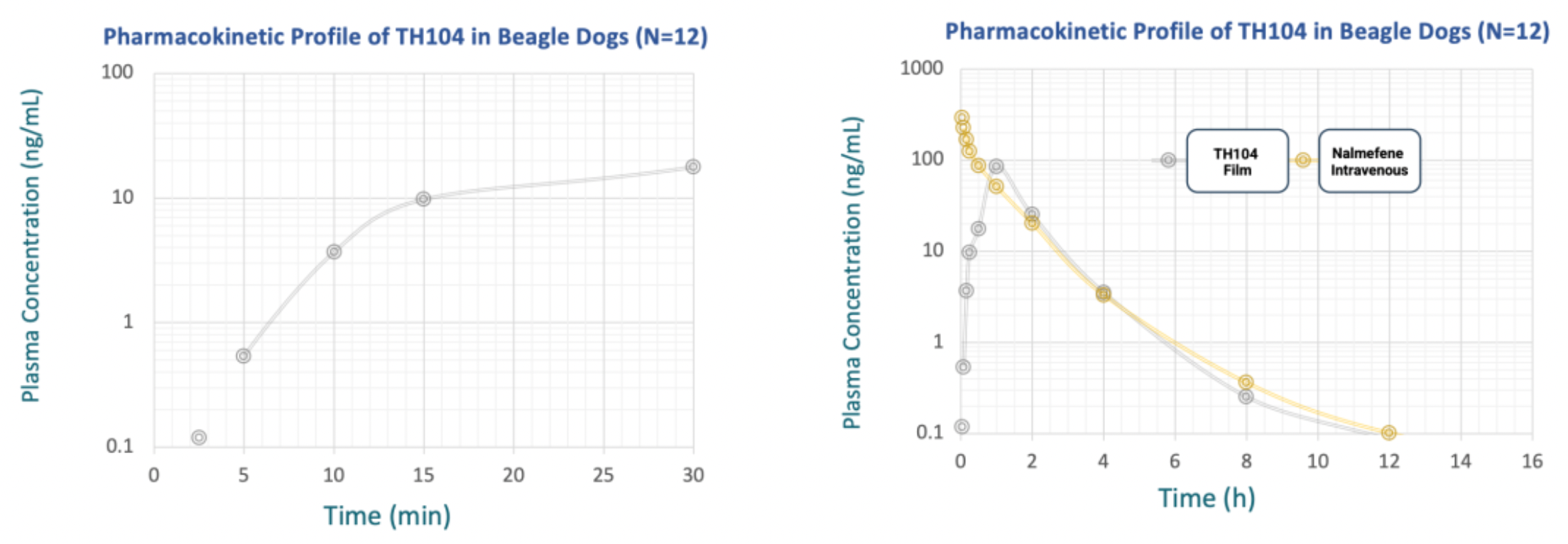
Clinical phase 1 studies conducted outside the United States indicate that TH104 is similar to the already marketed tablet drug nalmefene.
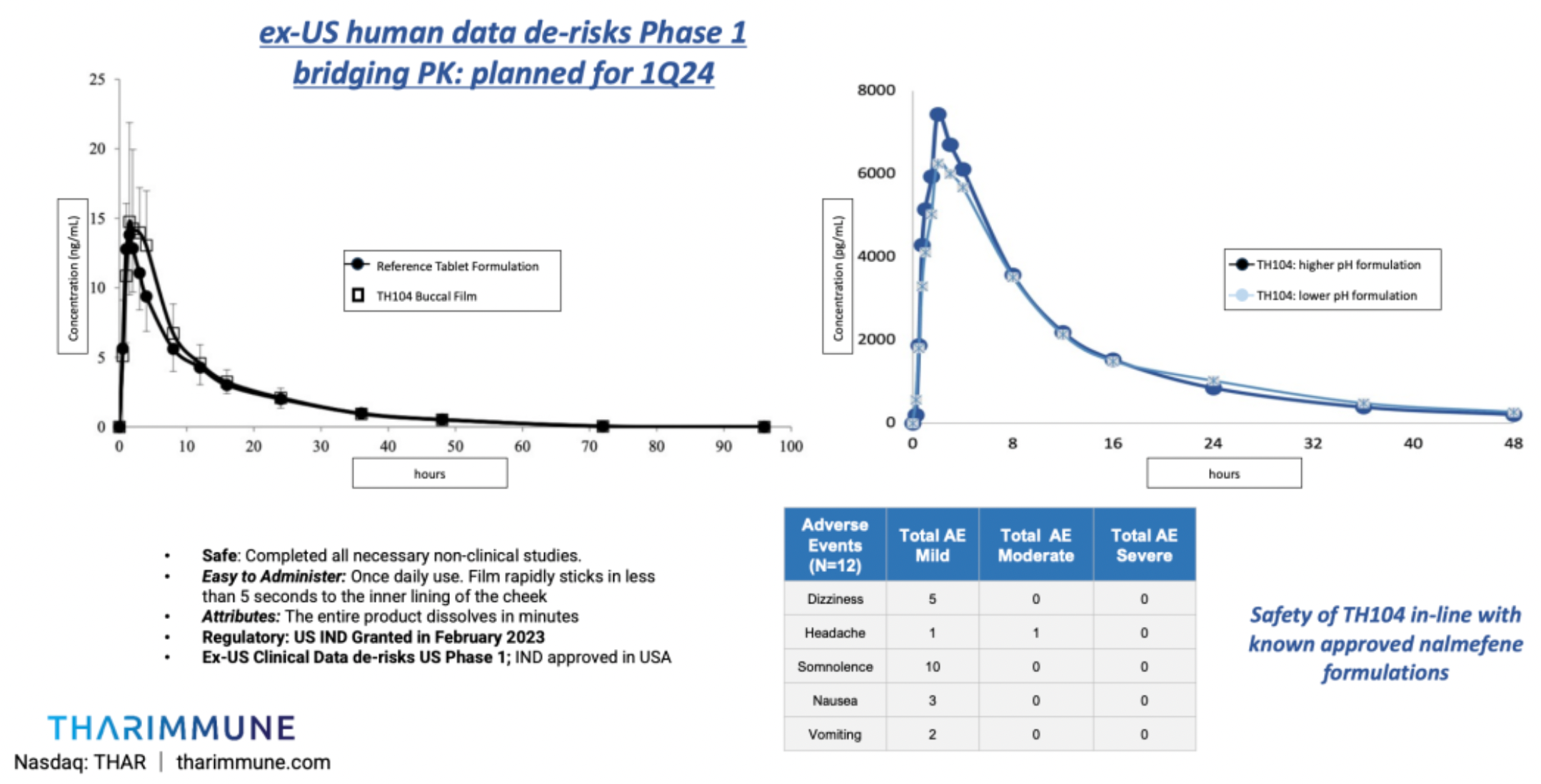
Clinical phase I studies conducted outside the United States have shown that 24 hours after administration, the average itch intensity in Group A and Group B decreased by 30.7% and 35.2%, respectively. All 12 participants experienced an average reduction of 33.3% in itch scores 24 hours after a single dose of TH104.
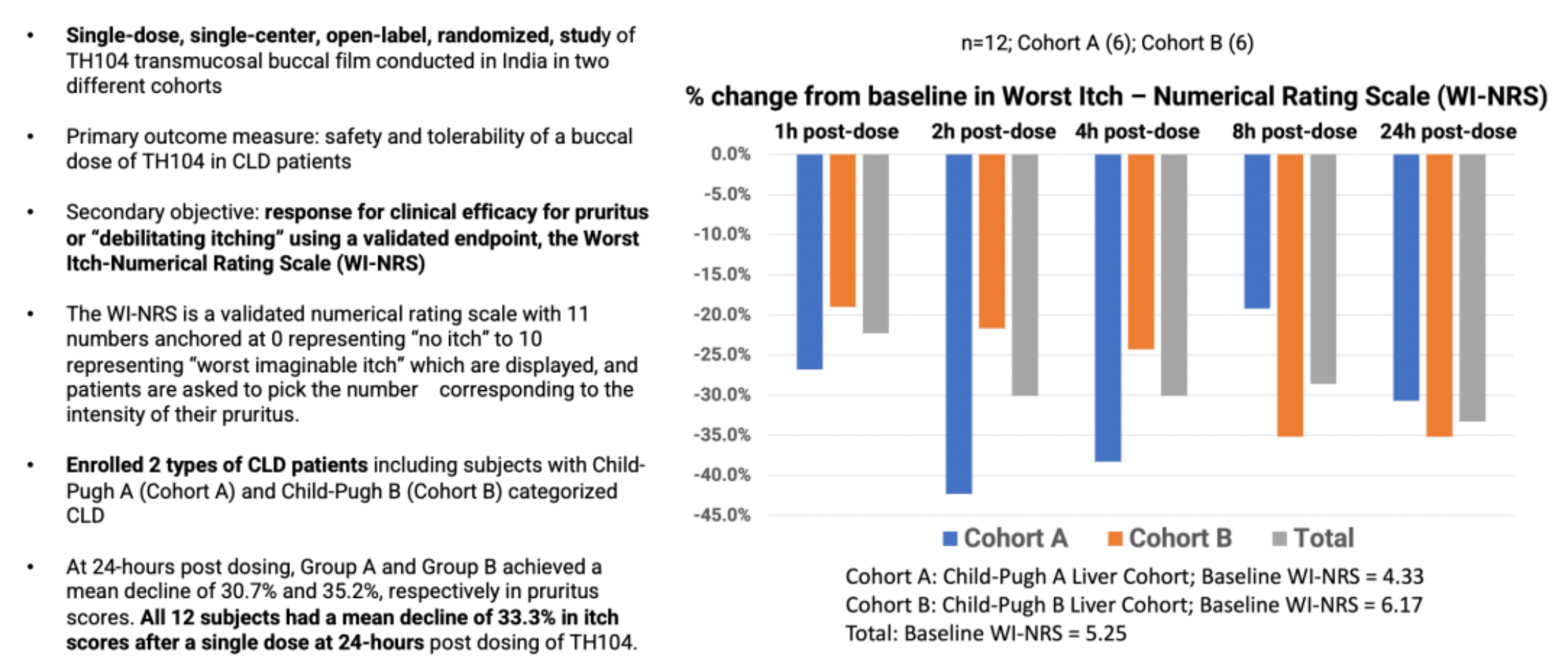
In terms of mechanism of action, in certain pruritic conditions (such as liver disease and atopic disorders), the itch cycle becomes dysregulated. TH104 primarily exerts its anti-pruritic effect by antagonizing two different subtypes of opioid receptors.
Primary Biliary Cholangitis (PBC)
Primary Biliary Cholangitis (PBC) is a chronic, progressive autoimmune liver disease that was previously known as Primary Biliary Cirrhosis. It primarily affects middle-aged women and is characterized by inflammation of the small bile ducts leading to progressive damage and fibrosis of the bile ducts, eventually resulting in diminished liver function.
In PBC, the body's immune system erroneously attacks and destroys the endothelial cells within the liver's small bile ducts responsible for transporting bile, leading to bile accumulation. This can cause a range of clinical symptoms, including fatigue, itching, jaundice, hyperpigmentation of the skin, and abnormal liver function, and may progress to cirrhosis or liver failure.
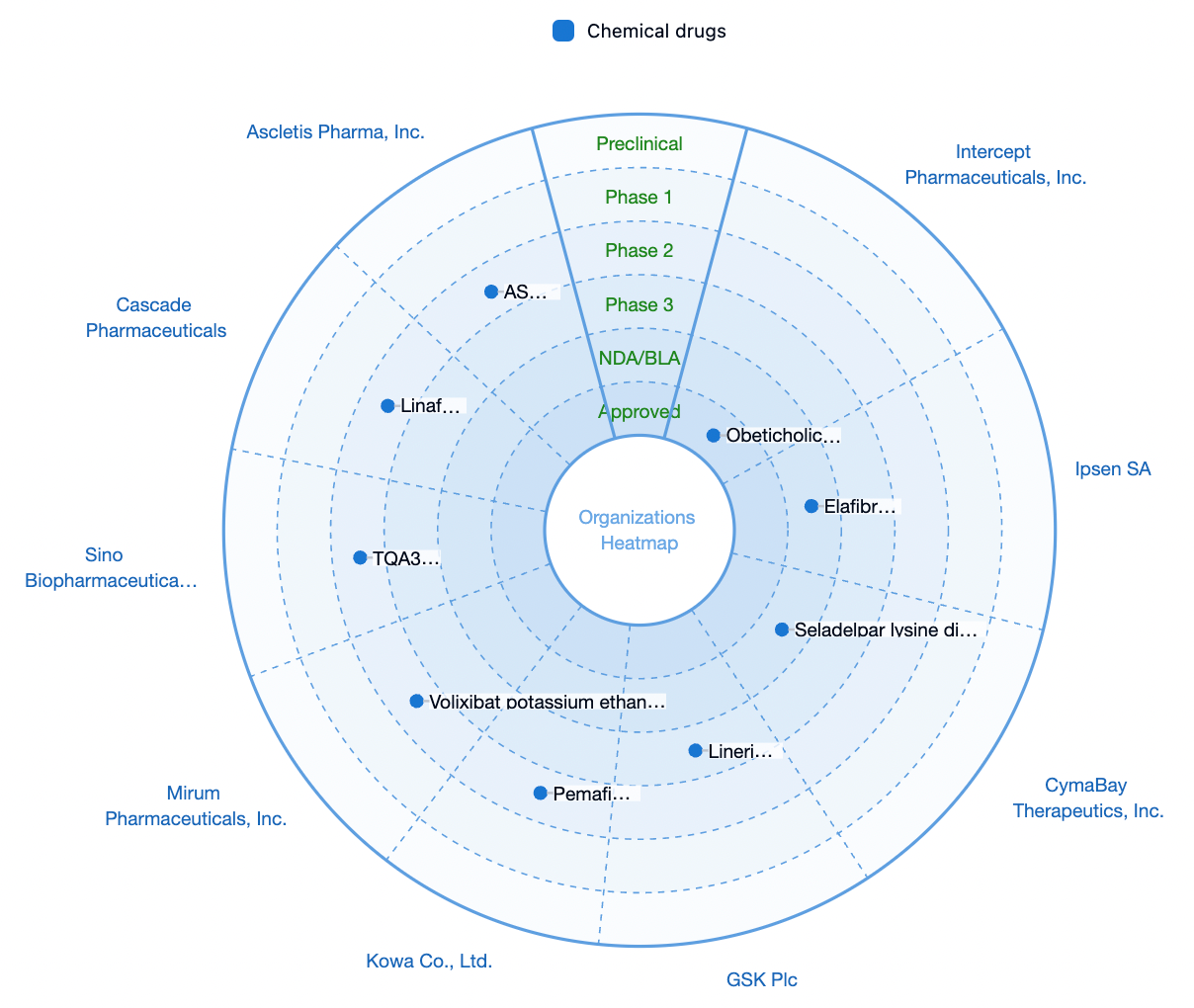
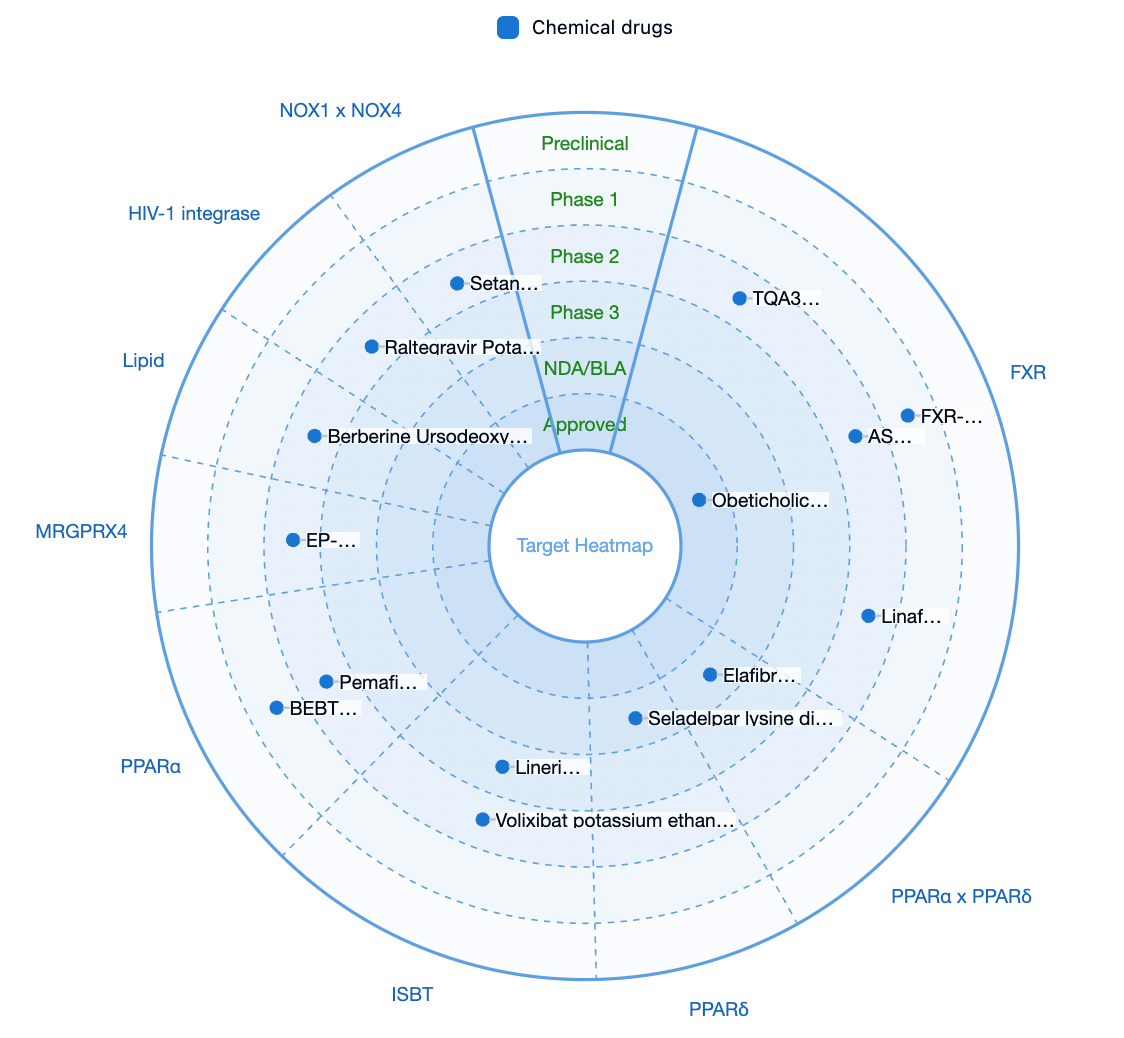
According to statistics from the Synapse database, currently, the only medication on the market approved for the treatment of PBC is obeticholic acid, a small molecule agonist targeting the farnesoid X receptor (FXR), developed by Intercept Pharmaceuticals and approved in 2016. Additionally, there are two products in the application stage for market approval and another one products that are in Phase III clinical trials.
Escient is developing an antagonist drug targeting MRGPRX4, which is intended for the treatment of cholestatic pruritus and cholestasis-like disorders, with its research pipeline EP547 having entered Phase 2 of clinical trials.
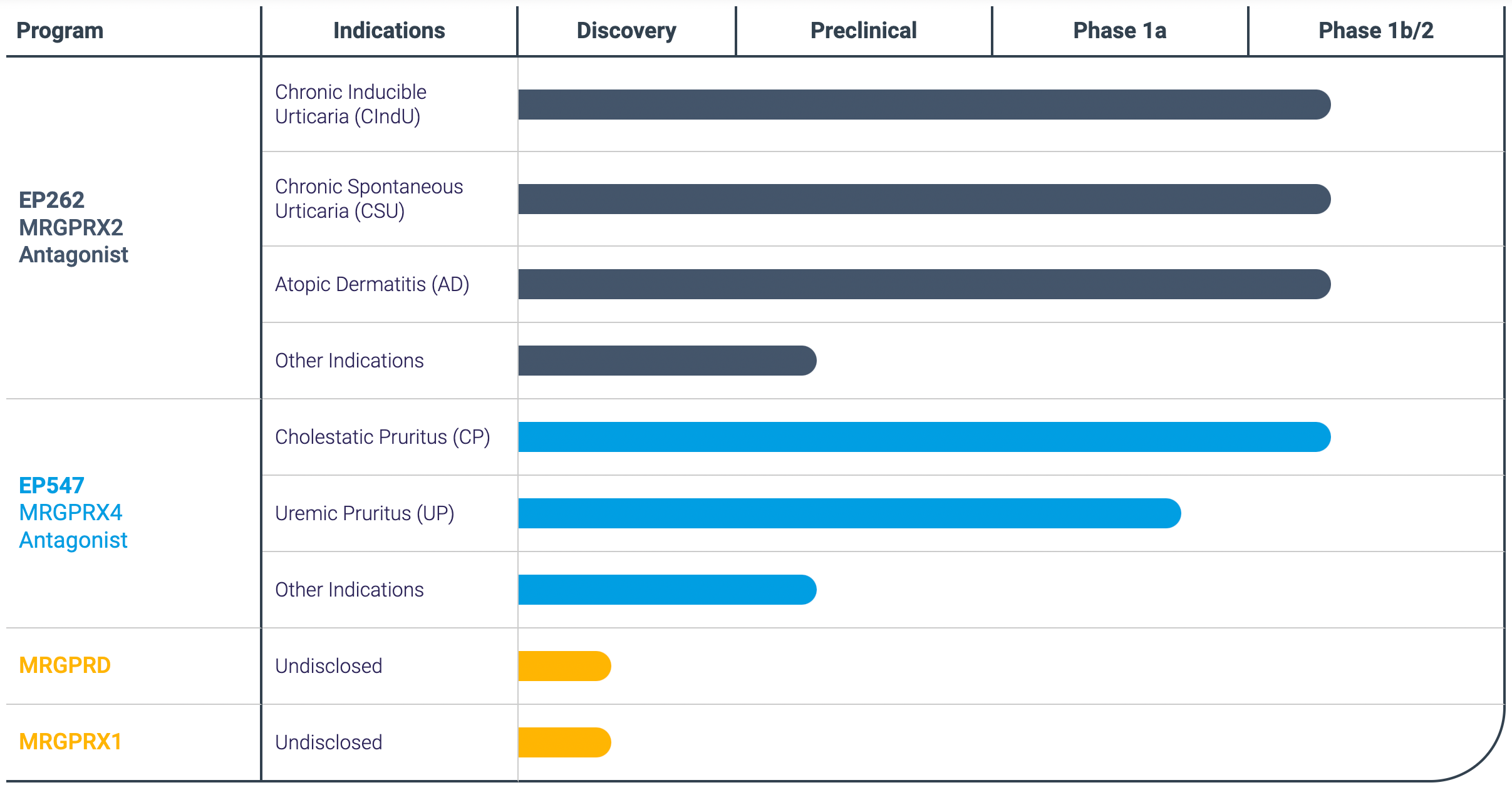
Escient also holds a leading position globally in the development of targets on MRGPRX4.