ProMIS Neurosciences begins Phase 1a trial, administering PMN310, a potential Alzheimer's treatment, to initial participants
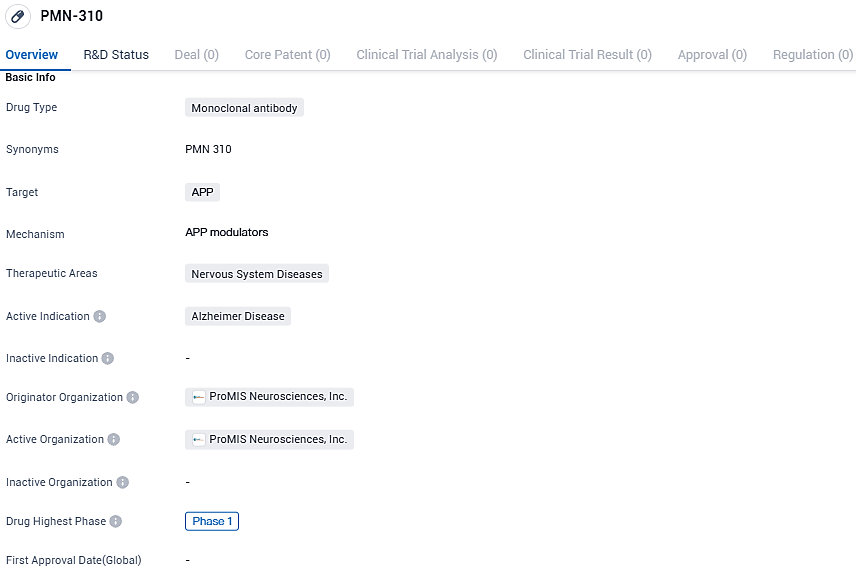
ProMIS Neurosciences Inc., a firm specializing in biotechnology, which is dedicated to the creation and growth of antibody treatments aimed at toxic misfolded proteins in neurodegenerative disorders like Alzheimer's disease, amyotrophic lateral sclerosis, and multiple system atrophy, has declared the commencement of their first human Phase 1a clinical trial for PMN310, a potential remedy for Alzheimer's disease, by administering the initial dosage to subjects.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
PMN310 is an innovative monoclonal antibody developed by the Company, designed to particularly target harmful amyloid-beta oligomers, considered to be a dominant instigator of AD. "We are enthusiastic about introducing our unique precision medicine method to the clinical world with the aim of crafting improved treatments for neurodegenerative diseases," stated Gail Farfel, Ph.D., the CEO of ProMIS Neurosciences.
"During Q3, we secured over 20 million dollars through private placement financing, which significantly solidifies our financial position and propels ProMIS towards attaining possibly value-enhancing milestones.” "We intend to incorporate up to five groups of eight healthy adult volunteers in this Phase 1a clinical trial, with each participant receiving one dosage of PMN310. We anticipate presenting the initial safety and pharmacokinetic data in the first half of the year 2024.
We are steadfast in examining our hypothesis that selectively targeting harmful Aβ oligomers - while circumventing monomer interference and plaque binding – may potentially provide differentiation in both safety and efficacy of AD treatment response.” "The outcomes from the Phase 1a research will aid in the selection of dosage for the subsequent Phase 1b research in patients with Mild Cognitive Impairment due to AD and patients with mild AD.
This research will utilize fresh findings from a third-party clinical study, which exhibited that plasma and cerebrospinal fluid efficacy biomarkers can show therapy response in as little as three months with oligomer-concentrated therapy.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
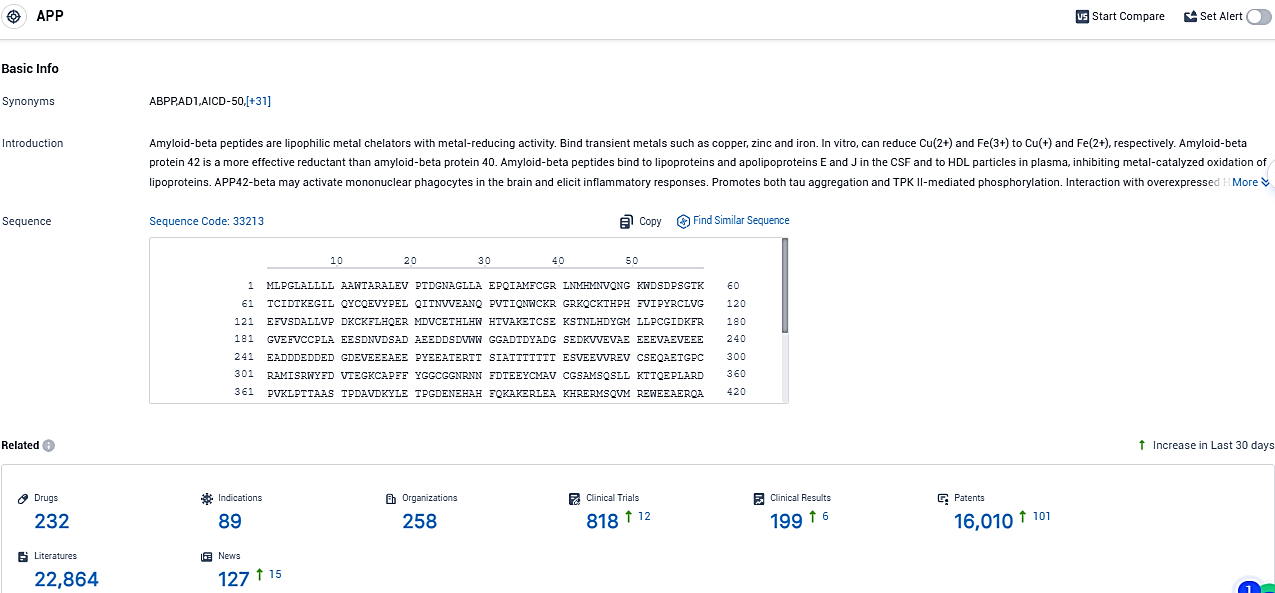
According to the data provided by the Synapse Database, As of November 28, 2023, there are 232 investigational drugs for the APP target, including 89 indications, 258 R&D institutions involved, with related clinical trials reaching 818, and as many as 16010 patents.
PMN-310 targets APP and is being developed for the treatment of Alzheimer's disease, a type of nervous system disease. As of now, it is in Phase 1 of clinical development, and further studies will be needed to determine its efficacy and safety.