Promising Early Results for BI-1206 in Chinese Non-Hodgkin's Lymphoma Study by CASI Pharma
CASI Pharmaceuticals, Inc. has disclosed promising initial results indicating effectiveness from the use of BI-1206 in conjunction with rituximab for individuals who are experiencing a recurrence or lack of response to previous treatments of indolent Non-Hodgkin’s Lymphoma, within its active research initiative in China.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
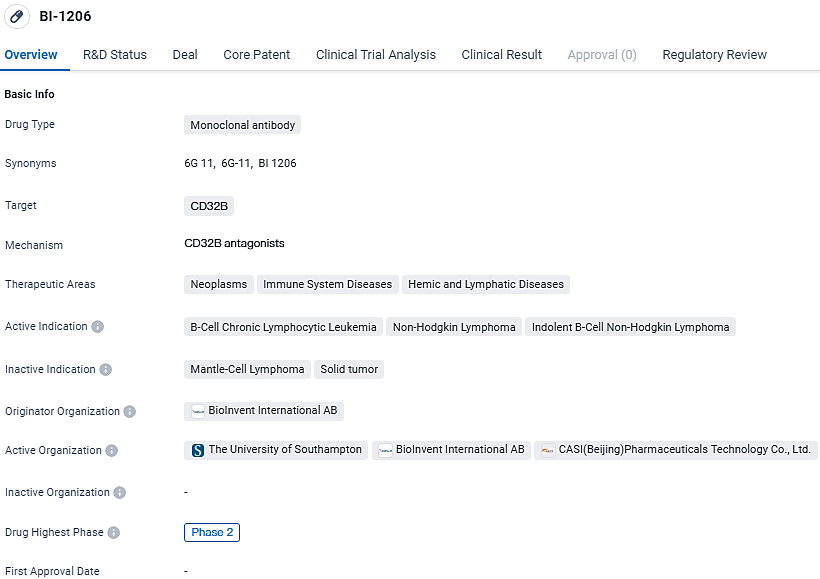
A novel fully human monoclonal antibody, BI-1206, is undergoing trials and has shown utility in blocking FcγRIIB. Current clinical investigations are exploring its use alongside rituximab for individuals suffering from refractory or relapsed indolent Non-Hodgkin Lymphoma (iNHL). The objective of the initial Phase 1 trial entails evaluating the antibody's safety profile, tolerability, pharmacokinetic properties, and its therapeutic effectiveness when delivered via intravenous infusion.
The early stages of the dose-finding Phase 1 trial revealed positive therapeutic indicators: among eight patients whose outcomes could be assessed, there were four instances of partial remission and one of full remission. These findings corroborate earlier clinical outcomes disclosed by BioInvent. Notably, within the trial demographic in China, a patient afflicted by relapsed Marginal Zone Lymphoma who achieved complete remission has shown sustained remission exceeding 20 weeks. These early findings indicate that BI-1206 is characterized by a safety profile that is well-tolerated by the study participants.
Dr. Wei-Wu He, the Executive at CASI Pharmaceuticals, expressed optimism based on the initial responses to BI-1206, highlighting the significance of the sustained and potent remissions observed at lower dosages of the medication. He expressed the sentiment that such results are crucial steps in corroborating BI-1206's efficacy for therapeutic use, thereby mitigating potential risks in the pharmaceutical's progression.
Dr. He elaborated on the mutual commitment between his company and BioInvent to advance the treatment avenues for iNHL with BI-1206. The encouraging interim findings have reinforced the decision to advance BI-1206 into further stages of clinical research as a therapeutic candidate for R/R iNHL.
On the same subject, Dr. Martin Welschof of BioInvent shared his insights, noting that BI-1206's development aims to renew the potency of existing cancer treatments like rituximab by counteracting inherent resistance mechanisms. The BI-1206 development trajectory is viewed with continued optimism, especially for applications in NHL, and updates on subsequent study findings are anticipated in the latter part of the year 2024.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
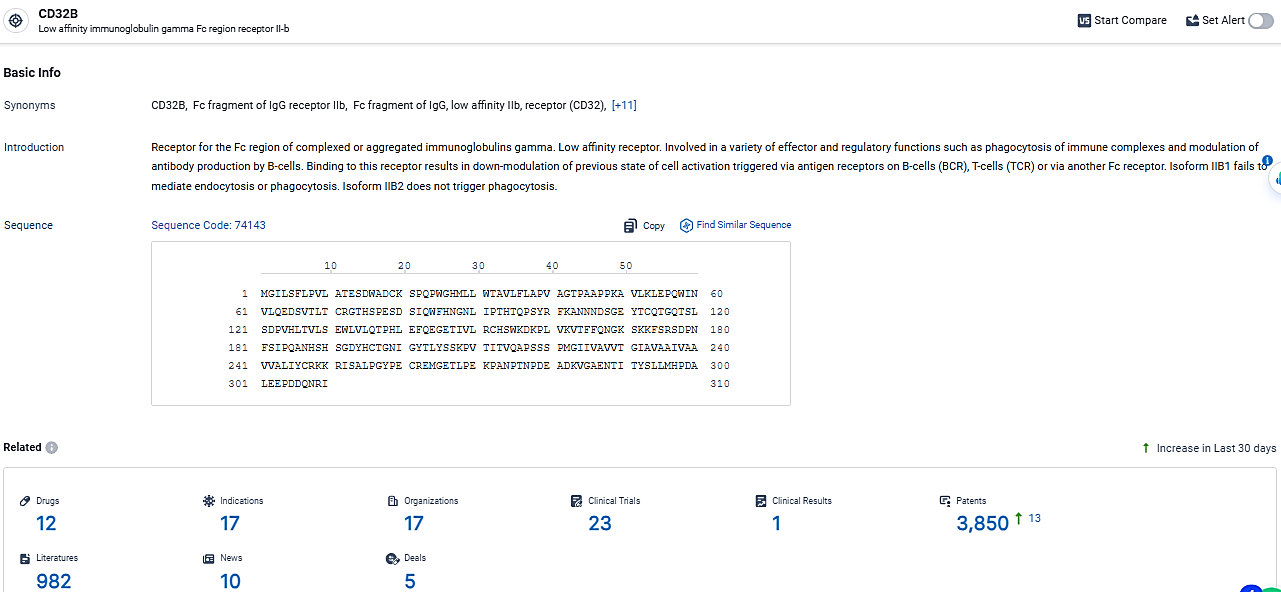
According to the data provided by the Synapse Database, As of March 6, 2024, there are 12 investigational drugs for the CD32B target, including 17 indications, 17 R&D institutions involved, with related clinical trials reaching 23, and as many as 3850 patents.
BI-1206 is a monoclonal antibody drug developed by CASI Pharmaceuticals, targeting CD32B. It is being investigated for its potential in treating B-cell chronic lymphocytic leukemia, non-Hodgkin lymphoma, and indolent B-cell non-Hodgkin lymphoma. The drug has reached Phase 2 globally and Phase 1 in China, indicating promising results in earlier stages of clinical development. Its orphan drug designation highlights its potential to address rare diseases, and its targeted mechanism of action suggests a potential for more precise and effective treatment.