Promising Phase 1/2 Results of Lexeo Therapeutics' LX2006 for Treating Friedreich Ataxia Cardiomyopathy
Lexeo Therapeutics, Inc., a company focused on genetic medicine and currently in the clinical stage, is committed to developing innovative therapies for cardiovascular diseases with a genetic basis and Alzheimer’s disease associated with the APOE4 gene. They have recently reported encouraging interim results for LX2006, which is being evaluated for its efficacy in treating cardiomyopathy in patients with Friedreich ataxia.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 Throughout both the Lexeo SUNRISE-FA Phase 1/2 clinical trial and the investigator-initiated Phase 1A trial at Weill Cornell Medicine, LX2006 demonstrated good tolerability without any treatment-related serious adverse events. Clinically significant enhancements in cardiac biomarkers were noted, showing progressive improvement over time.
Throughout both the Lexeo SUNRISE-FA Phase 1/2 clinical trial and the investigator-initiated Phase 1A trial at Weill Cornell Medicine, LX2006 demonstrated good tolerability without any treatment-related serious adverse events. Clinically significant enhancements in cardiac biomarkers were noted, showing progressive improvement over time.
“We are greatly heartened by these findings and the potential of LX2006 to address FA cardiomyopathy, a severe and fatal condition lacking approved therapies,” mentioned Dr. Eric Adler, Chief Medical Officer and Head of Research at Lexeo Therapeutics. “Considering the favorable safety profile and clinical benefits seen thus far, we are eager to pursue accelerated clinical development of LX2006, including the possibility of expedited approval for this potentially life-saving treatment.”
A recent subset analysis of natural history data conducted by Lexeo revealed increased left ventricular mass index (LVMI) in adults with FA cardiomyopathy. LVMI either remained stable or increased with age without spontaneous improvement. Higher LVMI indicates left ventricular hypertrophy and is linked with mortality in various cardiovascular disorders, including FA cardiomyopathy.
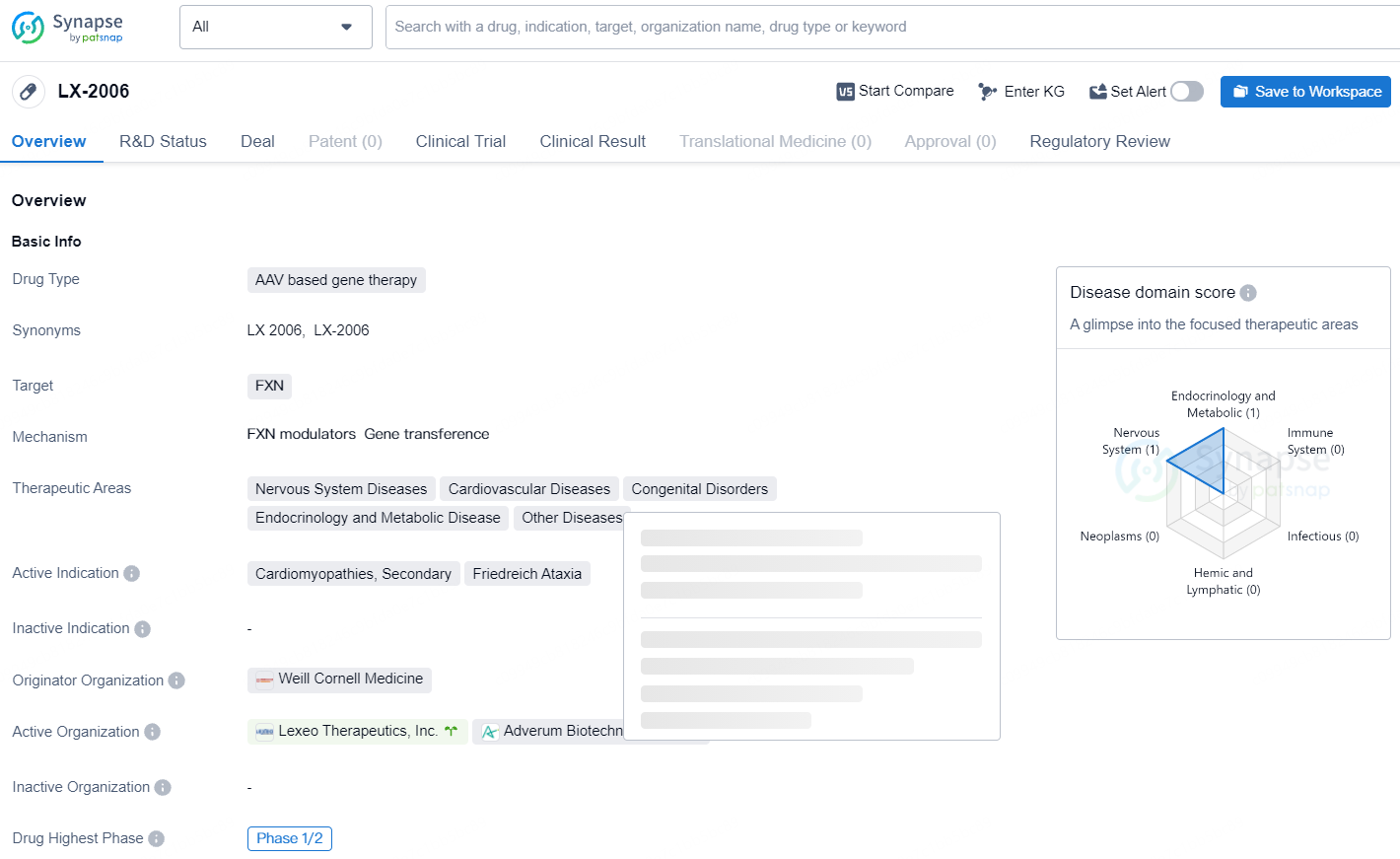
LX2006 is an intravenously administered AAV-based gene therapy candidate intended for the treatment of FA cardiomyopathy, the leading cause of death in individuals with FA, affecting around 5,000 people in the U.S. LX2006 aims to address the cardiac issues in FA by delivering a functional frataxin gene to stimulate frataxin protein production, thereby restoring mitochondrial function in heart cells.
In preclinical trials, LX2006 reversed cardiac abnormalities in FA disease models, showing improvements in cardiac function and survival while maintaining a good safety profile. The FDA has granted LX2006 Rare Pediatric Disease designation, Fast Track designation, and Orphan Drug designation for treating FA cardiomyopathy.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
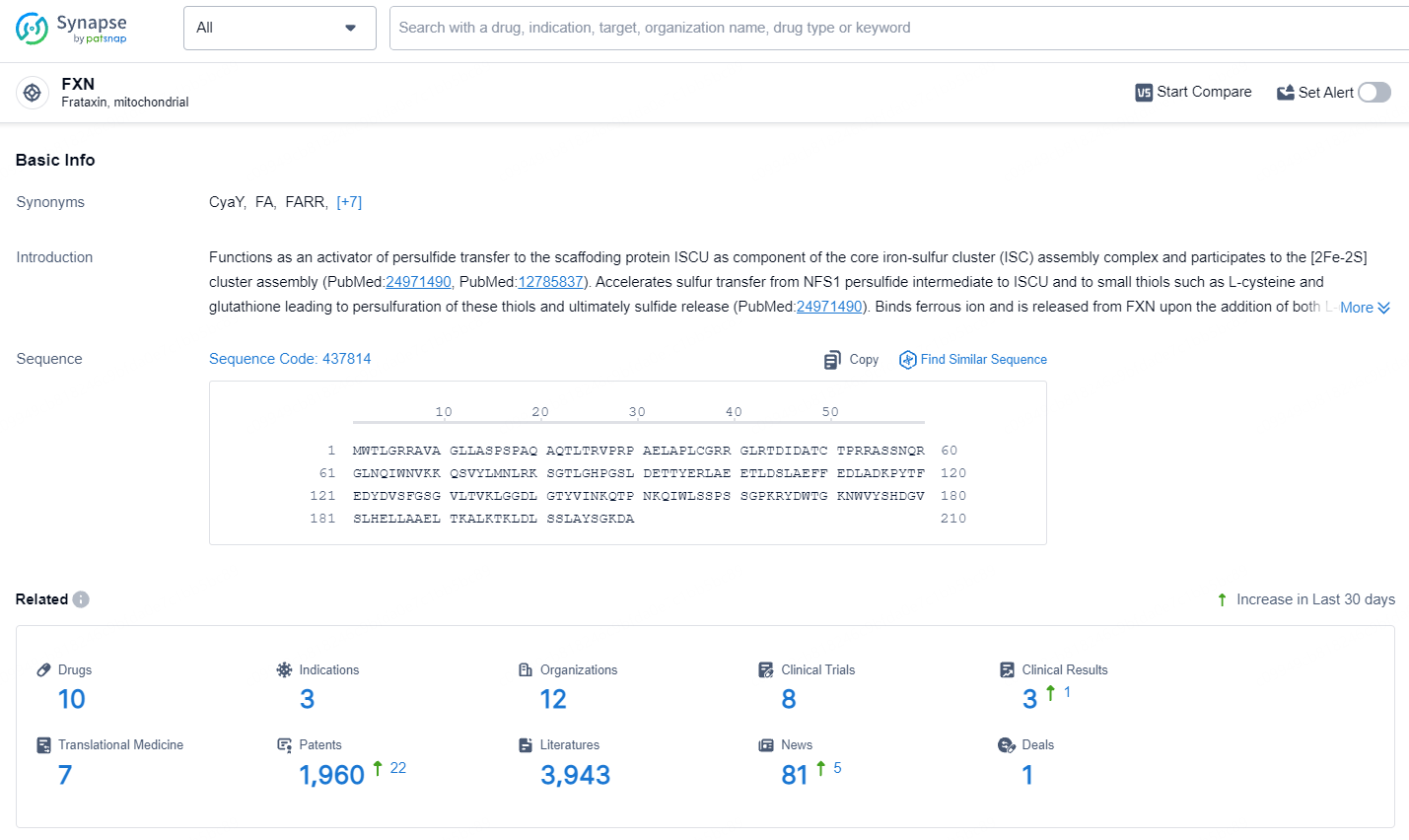
According to the data provided by the Synapse Database, As of July 17, 2024, there are 10 investigational drugs for the FXN target, including 3 indications, 12 R&D institutions involved, with related clinical trials reaching 8, and as many as 1960 patents.
LX-2006 targets the gene FXN and is being explored for the treatment of a variety of therapeutic areas including Nervous System Diseases, Cardiovascular Diseases, Congenital Disorders, Endocrinology and Metabolic Disease, and Other Diseases. With regulatory support through designations such as Rare Pediatric Disease, Fast Track, and Orphan Drug status, LX-2006 has the potential to bring much-needed treatment options to patients suffering from conditions such as Cardiomyopathies, Friedreich Ataxia, and other related diseases.