Promising Results for BTX-A51 in Liposarcoma Models: Edgewood Oncology's Trial Findings
Edgewood Oncology, a biotech firm in the clinical development phase, has revealed the release of novel early-stage research findings pertaining to the use of BTX-A51 in individuals with blood-related cancers and select solid tumors with specific genetic traits. This research on BTX-A51 was carried out in the context of human liposarcoma and is the collaborative effort of both the Dana-Farber Cancer Institute and the Hebrew University-Hadassah Medical School. The findings are expected to be showcased at the upcoming 2024 AACR Annual Meeting, set to take place in San Diego.
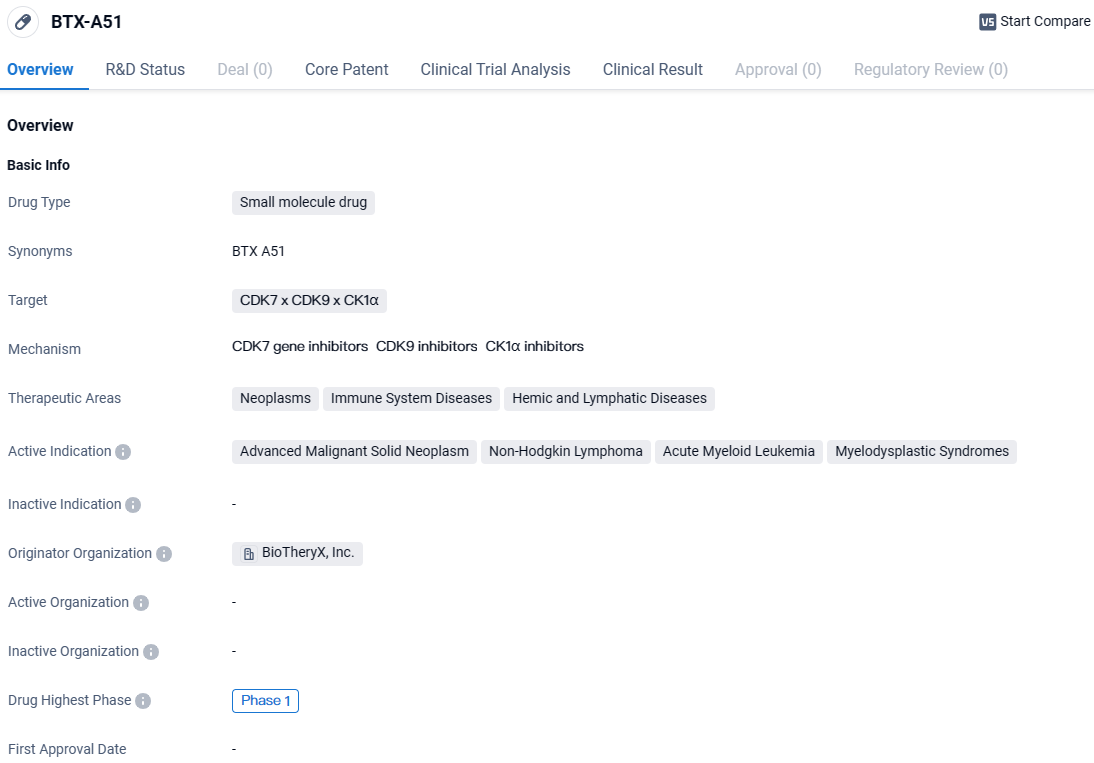
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
BTX-A51 stands out as a novel compound belonging to the category of compact molecule inhibitors that simultaneously disrupt several specific kinases, particularly casein kinase 1 alpha (CK1α) and crucial transcriptional modulators cyclin-dependent kinases 7 and 9 (CDK7 and CDK9). Its mechanism is designed to collectively suppress primary oncogenic controllers, which in turn initiates apoptotic processes in cancer cells.
During the session titled "Advancing Therapy by Inhibiting CK1α and Transcriptional CDKs (CDK7/9) for Treating Human Liposarcomas," research involving the use of BTX-A51 on preclinical human liposarcoma (LPS) models was showcased. Efficacy results from the use of BTX-A51 on LPS cell lines sourced from patients, as well as studies involving human xenografts, indicate a functional benefit. These studies further revealed how the dual inhibition of CK1α and CDK9 can work in tandem to enhance therapeutic outcomes.
Liposarcomas typically carry genetic mutations that result in the amplification of the mouse double minute 2 homolog (MDM2) gene, undermining normal p53 function. Research shows that treatment with BTX-A51 substantially diminishes MDM2 overactivity in several LPS cell lines derived from patients. This downregulation is followed by the reactivation of p53 protein levels, culminating in the induction of apoptosis. In practice, BTX-A51 has shown to be both tolerable and efficient in xenograft models derived from LPS patients.
David N. Cook, Ph.D., the CEO of Edgewood Oncology, expressed his satisfaction with the burgeoning interest from Dana-Farber researchers in BTX-A51, which is undergoing a Phase 2 clinical trial in combination with azacitidine for patients experiencing AML relapse or resistance. Dr. Cook further indicated that the recent findings underscore BTX-A51's potential applicability in oncological conditions with MDM2 gene amplifications, implying its effectiveness across a spectrum of cancers characterized by specific genetic alterations.
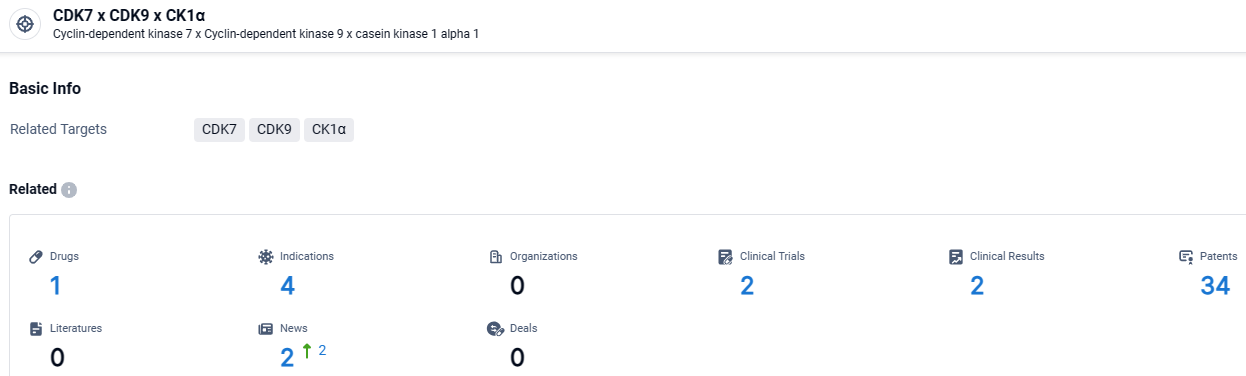
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 8, 2024, there are 1 investigational drugs for the CDK7 and CDK9 and CK1α target, including 4 indications, with related clinical trials reaching 2, and as many as 34 patents.
BTX-A51 falls under the therapeutic areas of neoplasms, immune system diseases, and hemic and lymphatic diseases. The drug targets CDK7, CDK9, and CK1α, which are proteins involved in cell cycle regulation and gene transcription. By targeting these proteins, BTX-A51 aims to inhibit their activity and potentially disrupt the growth and proliferation of cancer cells.