Promising Results for Nuvectis Pharma's NXP800 in ARID1a-Altered Ovarian Cancer
Nuvectis Pharma, Inc. has disclosed early-stage results from its continuous Phase 1b study evaluating NXP800 on individuals battling ovarian cancer that does not respond to platinum treatments and is characterized by mutations in the ARID1a gene. This condition represents a critical healthcare challenge with no adequate treatments currently available.
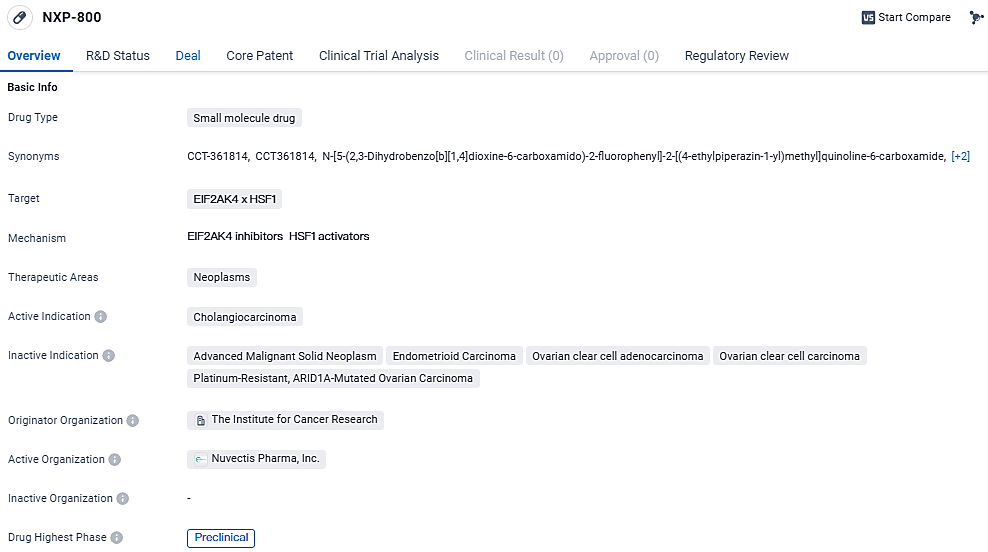
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The NXP800 initiative aimed at tackling this medical condition has earned the status of Fast Track Designation by the United States FDA. Currently, the program is facilitating a Phase 1b clinical trial, which includes premier medical research institutions across the US and UK.
The CEO of Nuvectis, Ron Bentsur, announced, "The preliminary findings from our Phase 1b trial of NXP800 in patients with ARID1a-mutated ovarian cancer who are resistant to platinum-based therapy are encouraging. The data so far shows a response rate of 33% and a disease control rate of 100% for the cohort evaluated for efficacy, where disease control is a combination of partial responses and maintained disease stability."
Mr. Bentsur added, "In addition to these findings, we have been refining the dosing protocols, comparable to strategies used with other potent therapeutic agents, to enhance our handling of significant side effects tied to the treatment with NXP800. Our team is optimistic that these refinements will lead to greater strides in the trial and will help realize the comprehensive capabilities of NXP800 for this particular clinical context."
The NXP800 molecule is administered orally and is a unique, potentially groundbreaking activator of the GCN2 kinase. Beyond the primary study, the Mayo Clinic is partnering in another research study evaluating NXP800's effectiveness against cholangiocarcinoma, another cancer type for which the FDA has awarded the drug Orphan Drug Designation.
The commitment to advancing NXP800 for ARID1a-mutated ovarian cancer patients who do not respond to platinum treatment has secured Fast Track Designation from the FDA. In the earlier part of 2023, NXP800 made headway with a positive conclusion of its Phase 1a dose-escalation study.
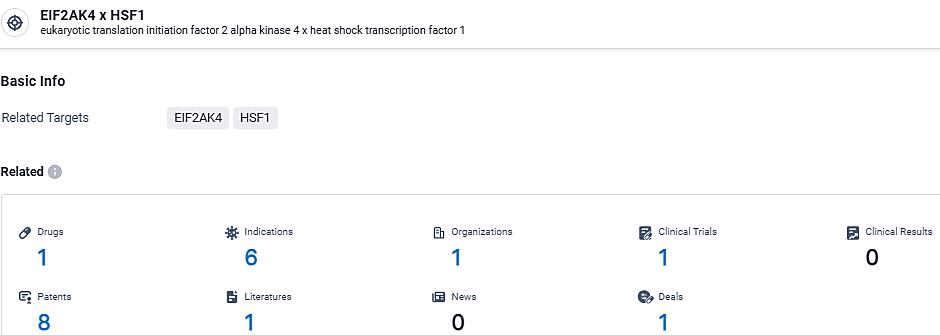
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 18 2024, there are 1 investigational drugs for the EIF2AK4 and HSF1 target, including 6 indications, 1 R&D institutions involved, with related clinical trials reaching 1, and as many as 8 patents.
NXP-800 is a small molecule drug that is being developed in the field of biomedicine. It targets EIF2AK4 and HSF1 and is primarily focused on treating neoplasms, specifically cholangiocarcinoma. The drug is currently in the preclinical phase, which means it is still undergoing laboratory testing and has not yet been tested on humans. NXP-800 has been designated as a Fast Track drug. This designation is given by regulatory authorities to drugs that show potential to address unmet medical needs and can expedite the development and review process.