Quell Therapeutics Advances CAR-Treg Therapy QEL-001 to Efficacy Phase in Liver Transplant Trial
Quell Therapeutics Ltd, a pioneering company in engineered T-regulatory cell treatments for severe immune-mediated diseases, is moving forward with QEL-001, their autologous engineered CAR-Treg cell therapy, into the efficacy phase of the LIBERATE Phase 1/2 trial for patients undergoing liver transplants. This progression comes after the successful administration in the initial safety phase and the subsequent approval by the trial's independent Data Safety and Monitoring Board, based on the review of clinical results.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
At the American Transplant Congress in Philadelphia, PA, USA, Professor Alberto Sánchez-Fueyo, a Hepatology Professor and Academic Director of the Institute of Liver Studies at Kings College London, as well as co-founder of Quell, shared updates on the LIBERATE study.
The preliminary findings advocate for continued research into QEL-001 CAR-Treg therapy for liver transplant patients with HLA-A2 mismatches. The aim is to promote operational tolerance and mitigate the adverse effects linked to immunosuppressive therapy.
Quell is now moving forward with an efficacy cohort to evaluate QEL-001 in liver transplant patients. This phase will measure tolerance induction at two and 12 months post complete cessation of IS therapy. The outcomes at two months are anticipated to be strong indicators of sustained operational tolerance.
Luke Devey, the Chief Medical Officer at Quell Therapeutics, remarked, "Entering the next stage of clinical trials with QEL-001 in liver transplantation is very promising. This trial is crucial for liver transplant recipients who otherwise face lifelong systemic immunosuppression with significant side effects, including higher risks of malignancies, infections, and renal failure, all of which severely affect their quality of life."
QEL-001 represents a pioneering, antigen-specific CAR-Treg cell therapy, utilizing Quell’s advanced multi-modular engineered Treg platform. The therapy encompasses three proprietary components: a chimeric antigen receptor for tissue targeting, the Foxp3 Phenotype Lock™ module, and a safety switch. The QEL-001 CAR targets HLA-A2, directing its action to the transplanted organ site in HLA-A2 mismatched liver transplant recipients.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
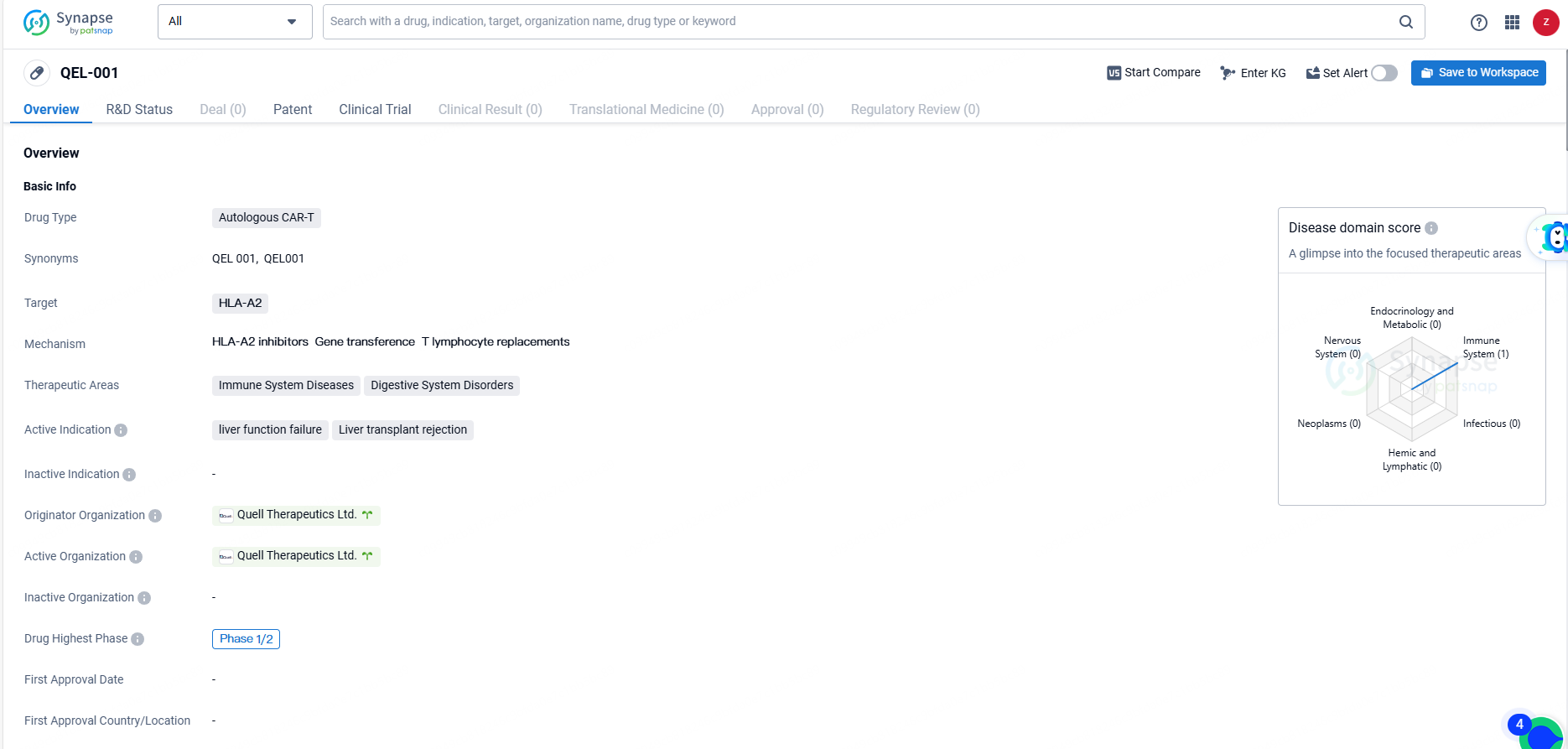
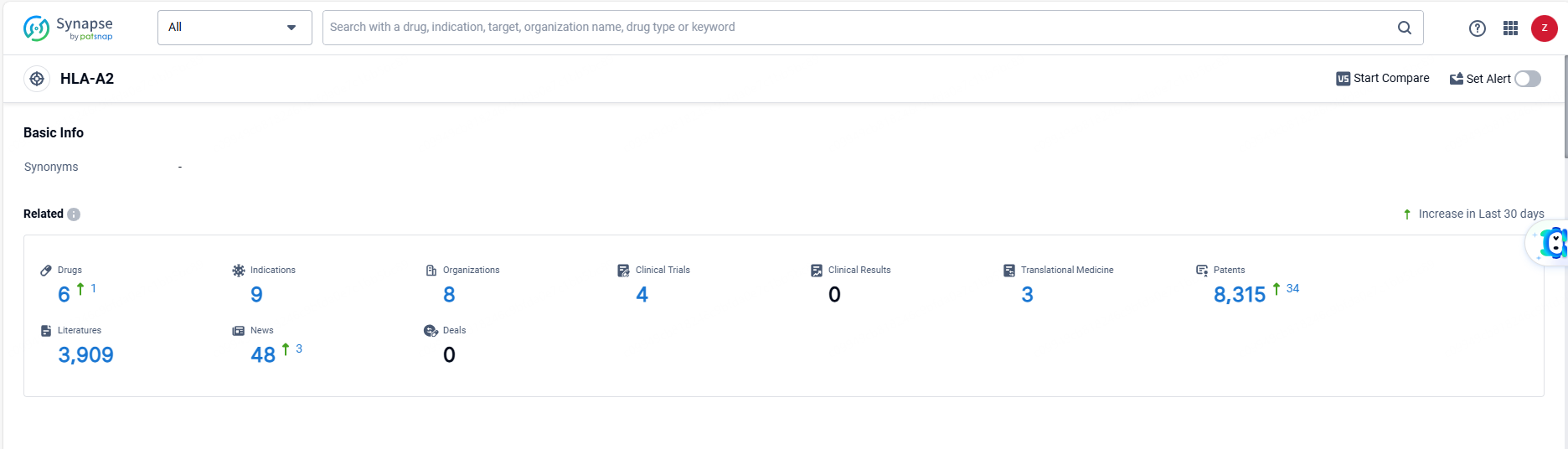
According to the data provided by the Synapse Database, As of June 13, 2024, there are 6 investigational drugs for the HLA-A2 target, including 9 indications, 8 R&D institutions involved, with related clinical trials reaching 4, and as many as 8315 patents.
QEL-001 targets HLA-A2 and is intended for the treatment of immune system diseases and digestive system disorders. The active indications for QEL-001 include liver function failure and liver transplant rejection. As of the latest available information, the drug has reached Phase 1/2 of clinical development.