Virion Therapeutics Reports Promising Phase 1b Results for HBV Treatment at EASL Congress
Virion Therapeutics, LLC, a biotechnology firm in the clinical stages of research, announced encouraging immunologic results along with ongoing safety and tolerability data from the inaugural human trial of their pioneering checkpoint modifier immunotherapy, VRON-0200, aimed at achieving a functional cure for HBV. The findings, presented by Professor Ed Gane, M.D., of the University of Auckland, were shared as a late-breaking abstract at the EASL Congress 2024 in Milan, Italy.
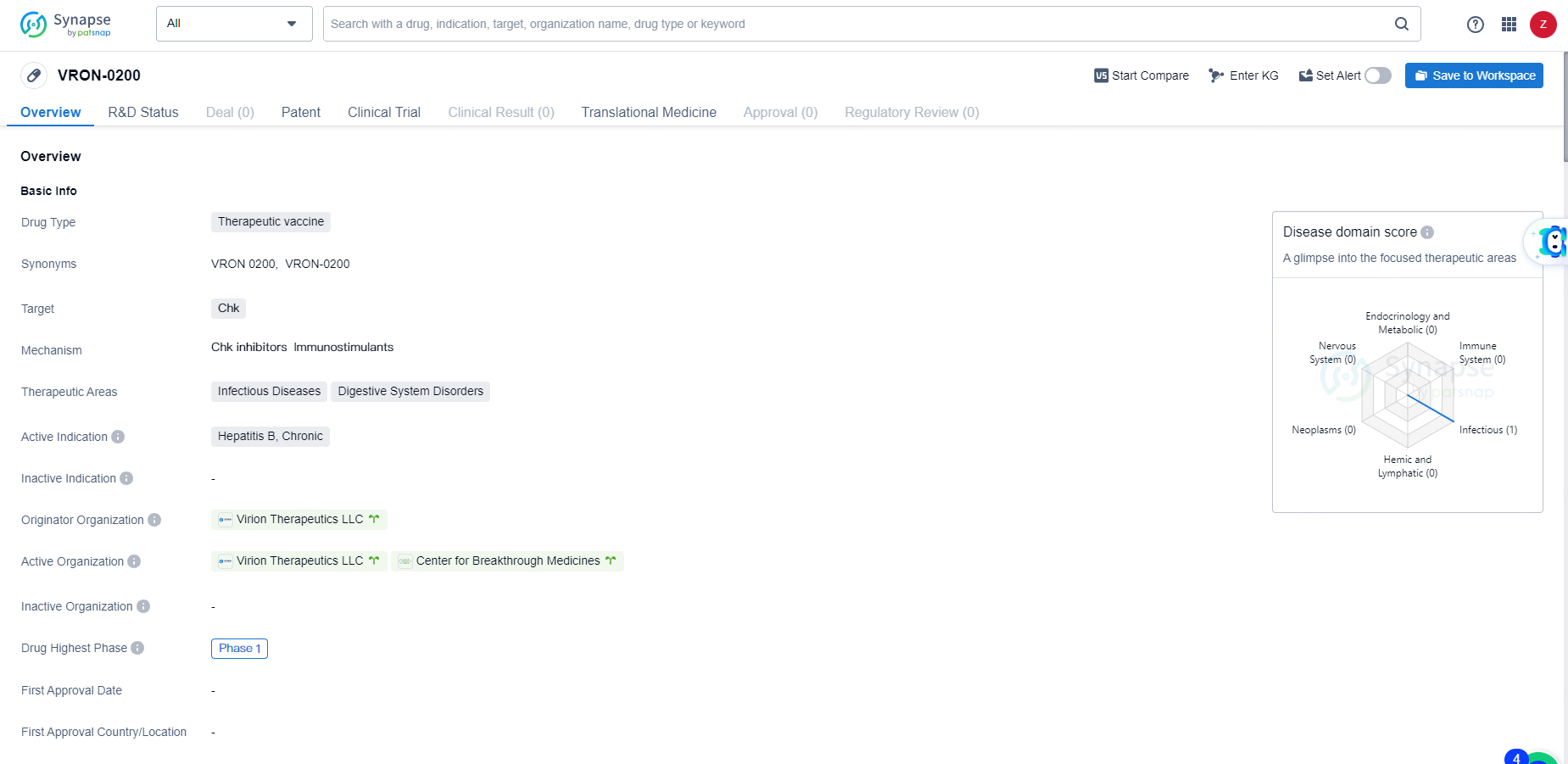
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The safety data from Phase 1b, involving the first 13 long-term hepatitis B patients on nucleos(t)ide antiviral therapy, all of whom received a single, low-dose, intramuscular administration of VRON-0200, indicated that the treatment was safe and well-tolerated. No significant adverse events or clinically relevant abnormalities, including in liver function tests, were reported. Among the 9 patients with at least 28 days of follow-up immunologic data, most showed enhanced T cell responses post-vaccination, with several maintaining responses above baseline values up to day 91.
Professor Gane remarked: “Individuals with chronic HBV infection experience immune exhaustion. Thus far, immunomodulatory therapies, including therapeutic vaccines, have not successfully stimulated HBV-specific T- and B-cell responses. These immunologic results are promising because a single intramuscular dose of VRON-0200 induced T cell responses in most patients, many of whom had minimal documented immunity before vaccination. Achieving T cell activation in this patient group, combined with a favorable safety profile and simple administration, is essential for potential HBV functional cure treatments. I am eager to see further data from this ongoing study."
"Immune-based treatments are now deemed necessary for achieving a functional cure for Chronic HBV. These data on VRON-0200—the first checkpoint modifier containing a T cell vaccine of any kind—demonstrated that a single low-dose vaccination can elicit immune responses in chronically HBV-infected patients. Following these positive safety and immunogenicity results, we are investigating further, evaluating VRON-0200 alone and in combination with other investigational agents, with announcements on these plans forthcoming,” stated Dr. Sue Currie, COO of Virion and one of the study's authors.
VRON-0200 is a therapeutic immunotherapy, administered intramuscularly, aimed at providing a functional cure for chronic HBV infection. While the virus itself triggers HBV-specific CD8+ T cells, patients who fail to clear the initial infection experience T cell exhaustion, limiting their proliferative capacity and viral control. Preclinical data support the hypothesis that VRON-0200, through checkpoint modification, can amplify, broaden, and enhance T cell responses, potentially activating T cells typically inactive during chronic HBV infection, thereby improving viral control.
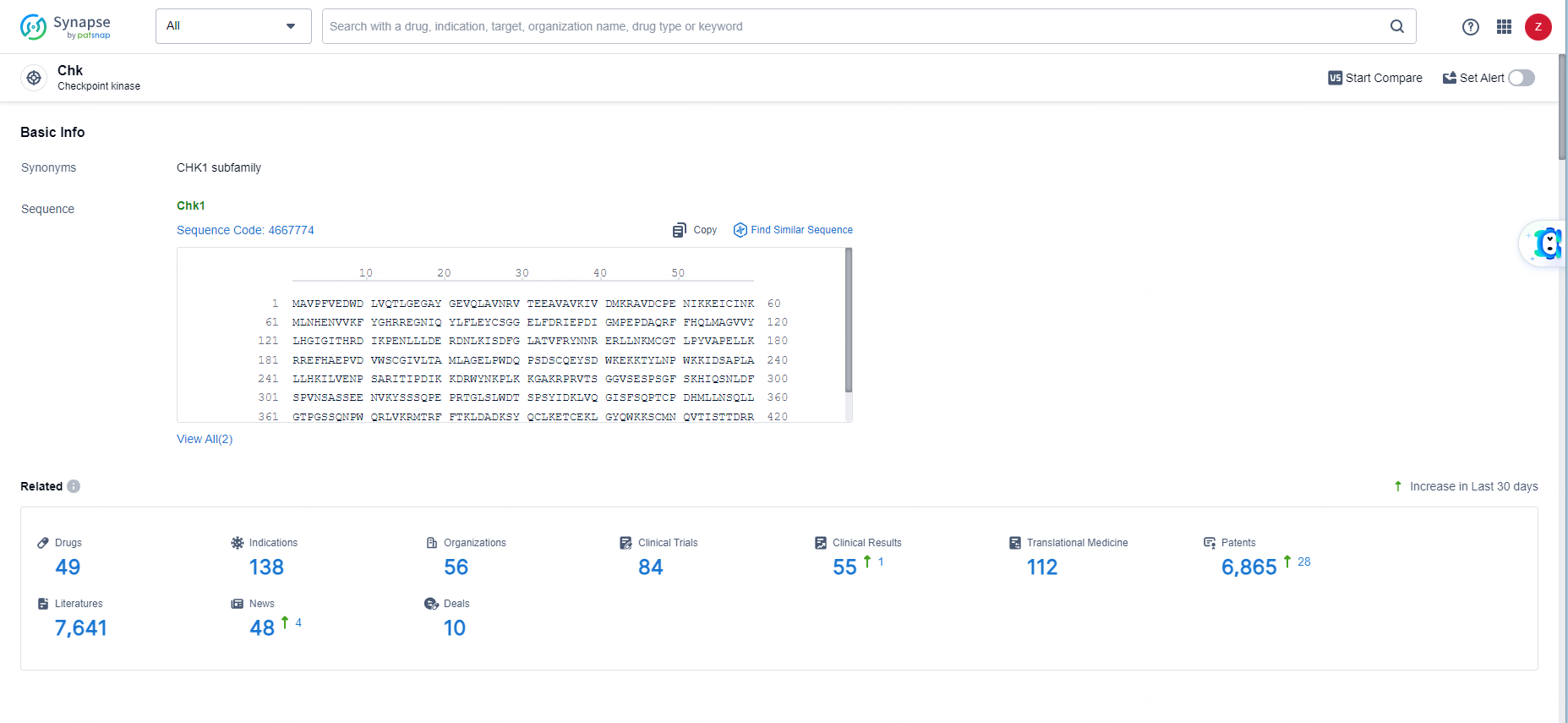
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 11, 2024, there are 49 investigational drugs for the Chk targets, including 138 indications, 56 R&D institutions involved, with related clinical trials reaching 84, and as many as 6865 patents.
VRON-0200 is a therapeutic vaccine targeting Chk for the treatment of chronic Hepatitis B. Its development represents a potential advancement in the field of biomedicine, particularly in addressing infectious diseases and digestive system disorders. As the drug progresses through clinical trials, further insights into its safety and efficacy will be gained, potentially offering new hope for patients with chronic Hepatitis B.