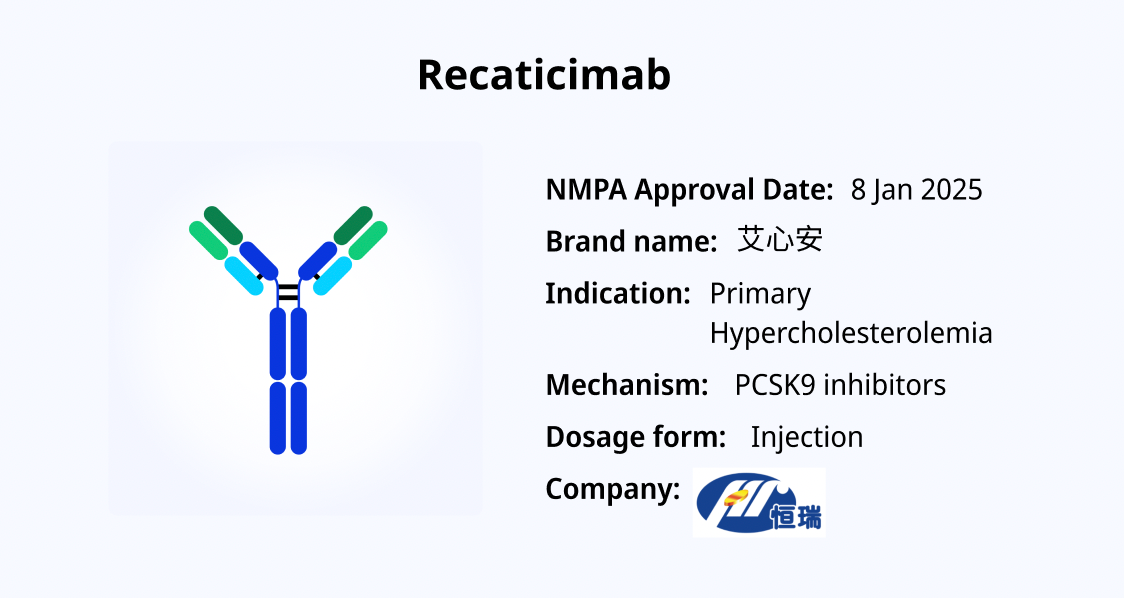
Recaticimab: A Novel PCSK9-Targeting Monoclonal Antibody for Hypercholesterolemia
Recaticimab, a monoclonal antibody developed by Hengrui Medicine, was approved by the National Medical Products Administration (NMPA) in China on January 8, 2025. Specifically targeting proprotein convertase subtilisin/kexin type 9 (PCSK9), this drug is designed for the treatment of hypercholesterolemia and other lipid disorders.
Mechanism of Action
PCSK9 is a liver-derived protein that plays a critical role in cholesterol metabolism by regulating the number of low-density lipoprotein receptors (LDLR) on the surface of liver cells. LDLR is responsible for clearing low-density lipoprotein cholesterol (LDL-C) from the bloodstream by binding to LDL particles and internalizing them into liver cells, where they are degraded into usable forms.
When PCSK9 binds to LDLR, the receptor is directed to the lysosomal degradation pathway instead of being recycled back to the cell surface. This reduces the availability of LDLR for LDL-C clearance, leading to increased LDL-C levels in the blood. Elevated LDL-C is a major risk factor for atherosclerosis, contributing to plaque formation in arterial walls and increasing the risk of cardiovascular diseases.
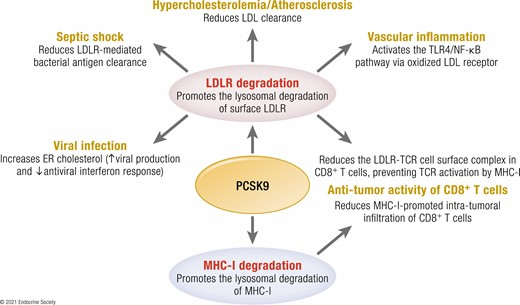
Recaticimab works by specifically binding to circulating PCSK9, preventing its interaction with LDLR. This allows more LDLR to remain active on liver cell surfaces, enhancing LDL-C clearance. Studies have shown that recaticimab significantly reduces levels of LDL-C, total cholesterol (TC), non-high-density lipoprotein cholesterol (non-HDL-C), apolipoprotein B (ApoB), and lipoprotein(a) [Lp(a)].
Drug Characteristics
Recaticimab has undergone molecular optimization, featuring a preferred IgG1 protein subtype and incorporating "YTE" amino acid mutation technology. This design enhances the drug’s specificity and affinity for PCSK9 while significantly extending its half-life in vivo. As a result, patients can benefit from extended dosing intervals, such as every 4 weeks or 8 weeks, with a maximum possible interval of 12 weeks.
Recaticimab is indicated for adult patients with primary hypercholesterolemia (including both heterozygous familial and non-familial hypercholesterolemia) and mixed dyslipidemia who have not achieved their target LDL-C levels despite receiving moderate to high-intensity statin therapy. Additionally, it can be used as monotherapy for patients who are either intolerant to or unwilling to use statins.
The approved dosing regimen for recaticimab includes a 150 mg dose every 4 weeks (Q4W) and a 300 mg dose every 8 weeks (Q8W). Compared to other PCSK9 inhibitors that require more frequent injections, recaticimab offers an extended dosing interval, which improves patient adherence. The incorporation of YTE amino acid mutation technology further prolongs its half-life, enhancing treatment convenience and efficacy.
By reducing the need for frequent injections, recaticimab significantly improves patient adherence and minimizes the burden of frequent administration, thereby enhancing overall quality of life. Additionally, its flexible dosing regimen allows physicians to tailor treatment according to individual patient needs, supporting a more personalized approach to managing hypercholesterolemia and lipid disorders.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Refrence
- 1. Seidah NG, Prat A. The Multifaceted Biology of PCSK9. Endocr Rev. 2022 May 12;43(3):558-582. doi: 10.1210/endrev/bnab035. PMID: 35552680; PMCID: PMC9113161.