REGENXBIO Study in The Lancet: Single-Dose Gene Therapy ABBV-RGX-314 for Wet AMD
REGENXBIO Inc. has publicized the findings from their Phase I/IIa study, which assessed the safety profile and the degree of acceptability of one administration of ABBV-RGX-314 delivered subretinally to treat patients with neovascular age-related macular degeneration.
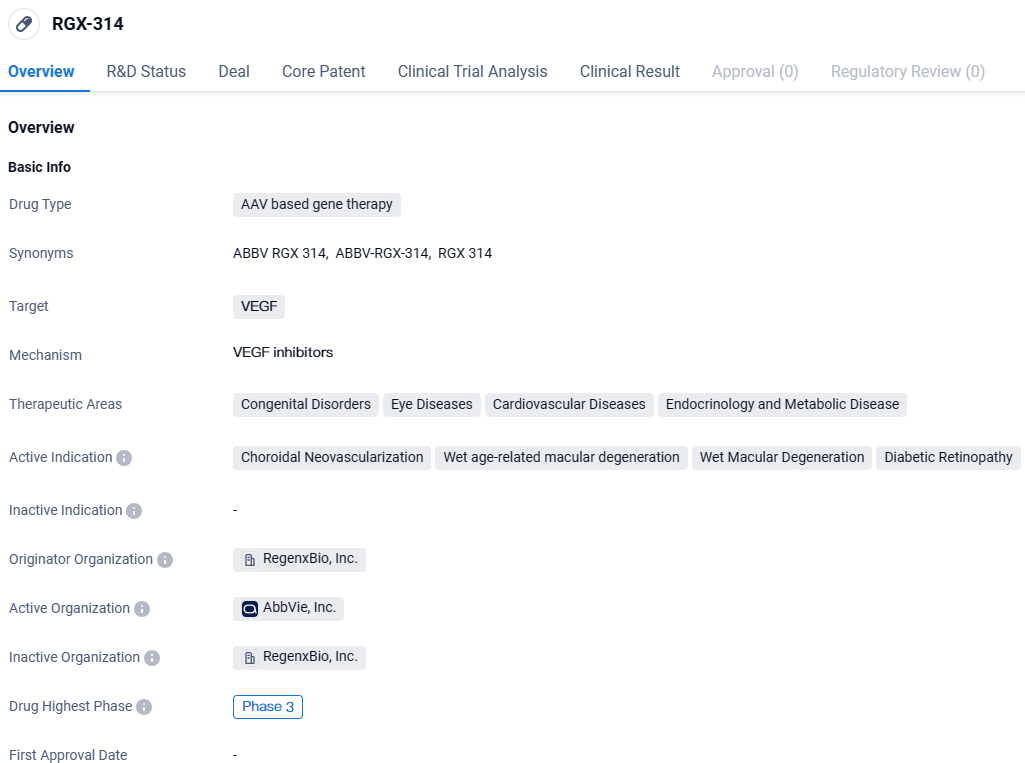
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Research findings spanning two years were disclosed in a noteworthy article named “Gene therapy for neovascular age-related macular degeneration by subretinal delivery of RGX-314: a phase 1/2a dose-escalation study,” documented in The Lancet journal. These encouraging outcomes have propelled further clinical studies, specifically the advanced-stage evaluations of ABBV-RGX-314 as a promising single-dose gene therapy to address wet AMD.
Kenneth T. Mills, the CEO and President of REGENXBIO, announced at the outset of 2024, "The ABBV-RGX-314 program has kicked off the year with robust and favorable data. We have high expectations that this compound could not only emerge as the pioneering gene therapy for wet AMD but also transform into the preferred treatment option, shaping the future of diabetic retinopathy care with multi-billion-dollar market implications.”
According to the details that were featured in The Lancet, administering ABBV-RGX-314 once was predominantly met with acceptability from a safety perspective. Most subjects experienced consistent or improved states regarding visual acuity and the thickness of their retina, with the majority requiring minimal or no additional anti-VEGF treatments after the two-year mark. Subjects in the study who were administered appropriate doses of ABBV-RGX-314 showed consistent levels of the protein linked to this therapy. They also displayed maintained or enhanced visual and retinal health, with a negligible need for any extra anti-VEGF treatments, as observed over a period extending to two years.
Moreover, REGENXBIO released supplementary encouraging provisional outcomes from an ongoing extended-term surveillance study on ABBV-RGX-314. The additional data suggest the enduring tolerability and effectiveness of this therapy, with sustained effects observed for up to a four-year period.
ABBV-RGX-314 is under examination in patients with wet AMD within two critical trials titled ATMOSPHERE and ASCENT™. The pace of participant enrollment is aligned with expectations, and the outcomes from these studies are anticipated to underpin a series of regulatory filing submissions with both the U.S. Food and Drug Administration and the European Medicines Agency, projected for late 2025 into the first half of 2026.
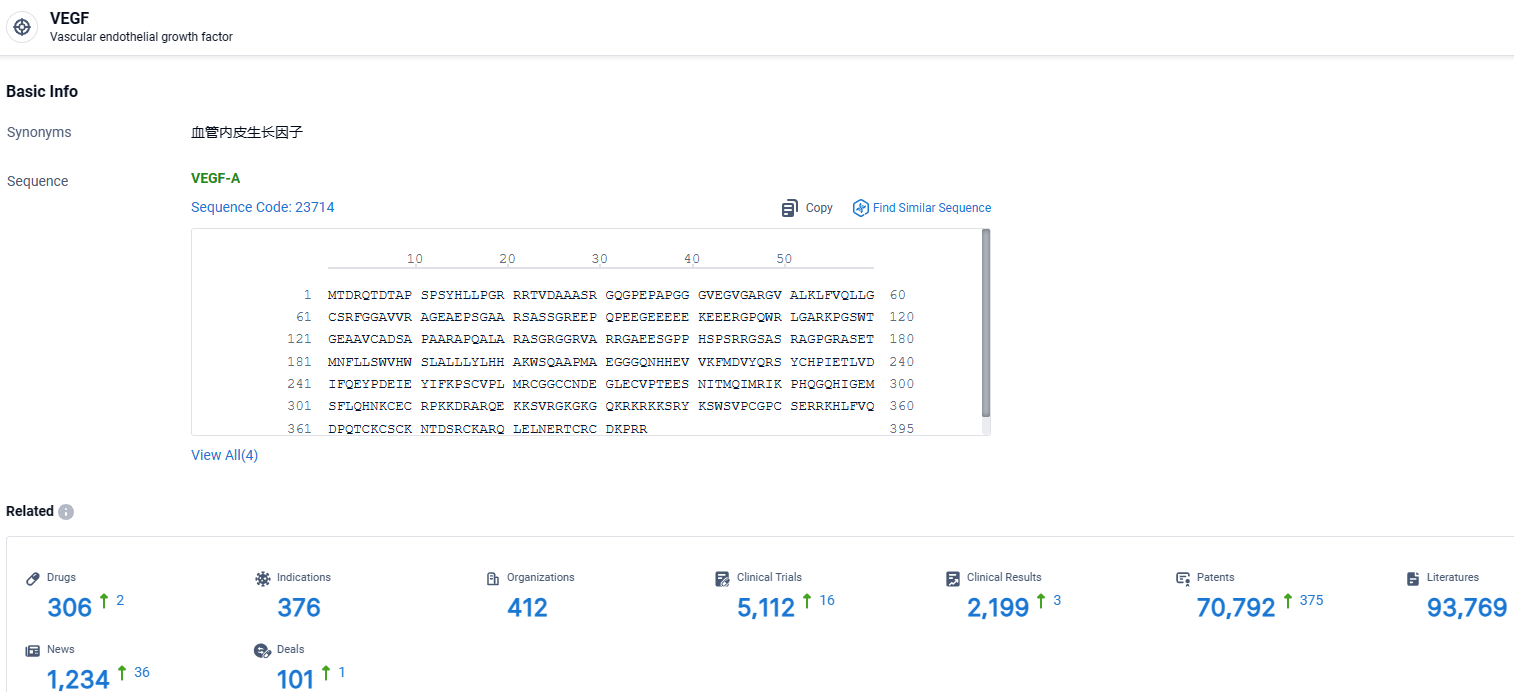
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 1, 2024, there are 306 investigational drugs for the VEGF target, including 376 indications, 412 R&D institutions involved, with related clinical trials reaching 5112, and as many as 70792 patents.
RGX-314 targets VEGF and is primarily focused on treating various eye diseases, including choroidal neovascularization, wet age-related macular degeneration, wet macular degeneration, and diabetic retinopathy. The drug is currently in Phase 3, the highest phase of clinical development, indicating its potential for commercial use in the future.