Relaxin Receptors: Therapeutic Opportunity for Heart Failure and Pulmonary Arterial Hypertension
On November 28, 2023, Tectonic Therapeutic, a biotech company dedicated to discovering novel GPCR-targeted therapies, announced the completion of dosing in the first patient enrolled for their Fc-relaxin fusion program targeting the RXFP1 receptor. Tectonic Therapeutic's TX000045 Fc-relaxin fusion protein, referred to as "TX45," is a potentially best-in-class formulation that, through protein engineering efforts, has overcome the biophysical limitations of human hormones, delivering optimal pharmacokinetics, target engagement, and developability. Following the completion of a dose-escalation safety study in healthy volunteers, the continued development of TX45 will focus on high unmet needs within cardiopulmonary indications.

As an agonist for the relaxin-1 receptor (RXFP1), relaxin produces unique and diverse biological effects including pulmonary and systemic vasodilation, tissue remodeling/fibrosis reversal, and inflammation reduction. Owing to these characteristics, it has the potential for significant therapeutic effects in cardiopulmonary diseases. Additionally, it is also referred to as the "pregnancy hormone" as it is upregulated during pregnancy to help the cardiovascular system of expectant mothers meet the increasing demands of the developing fetus and to remodel tissues and musculoskeletal structures.
Tectonic Therapeutics was co-founded in 2019 by Andrew Kruse and Tim Springer from Harvard Medical School, dedicated to transforming the discovery of antibodies and other biologics targeting GPCRs (G protein-coupled receptors). Building on the pioneering work from Dr. Kruse's laboratory, Tectonic Therapeutics' proprietary GEODe platform has overcome challenges previously encountered in identifying biologics that modulate GPCR signaling, thereby advancing the development of novel GPCR-targeted therapies.
About Relaxin Receptors
The Relaxin family peptide receptors (RXFP) consist of two members in the human body, RXFP1 and RXFP2, and are part of the leucine-rich repeat-containing G protein-coupled receptors (LGRs). LGRs are involved in various processes of reproductive and developmental biology. RXFP1 is the receptor for human relaxin-2 hormone and plays a crucial role during pregnancy. It is responsible for physiological changes, including increased cardiac output and remodeling of reproductive tissues to facilitate childbirth. RXFP1 signaling also regulates the physiological functions of many organs in both males and females, particularly the heart, lungs, liver, and kidneys. In these organs, the activation of RXFP1 by relaxin-2 leads to multiple cellular effects, including vasodilation, angiogenesis, anti-inflammatory responses, and remodeling of the extracellular matrix through collagen degradation. As a result, RXFP1 has emerged as a promising therapeutic target for the treatment of cardiovascular and fibrotic diseases.
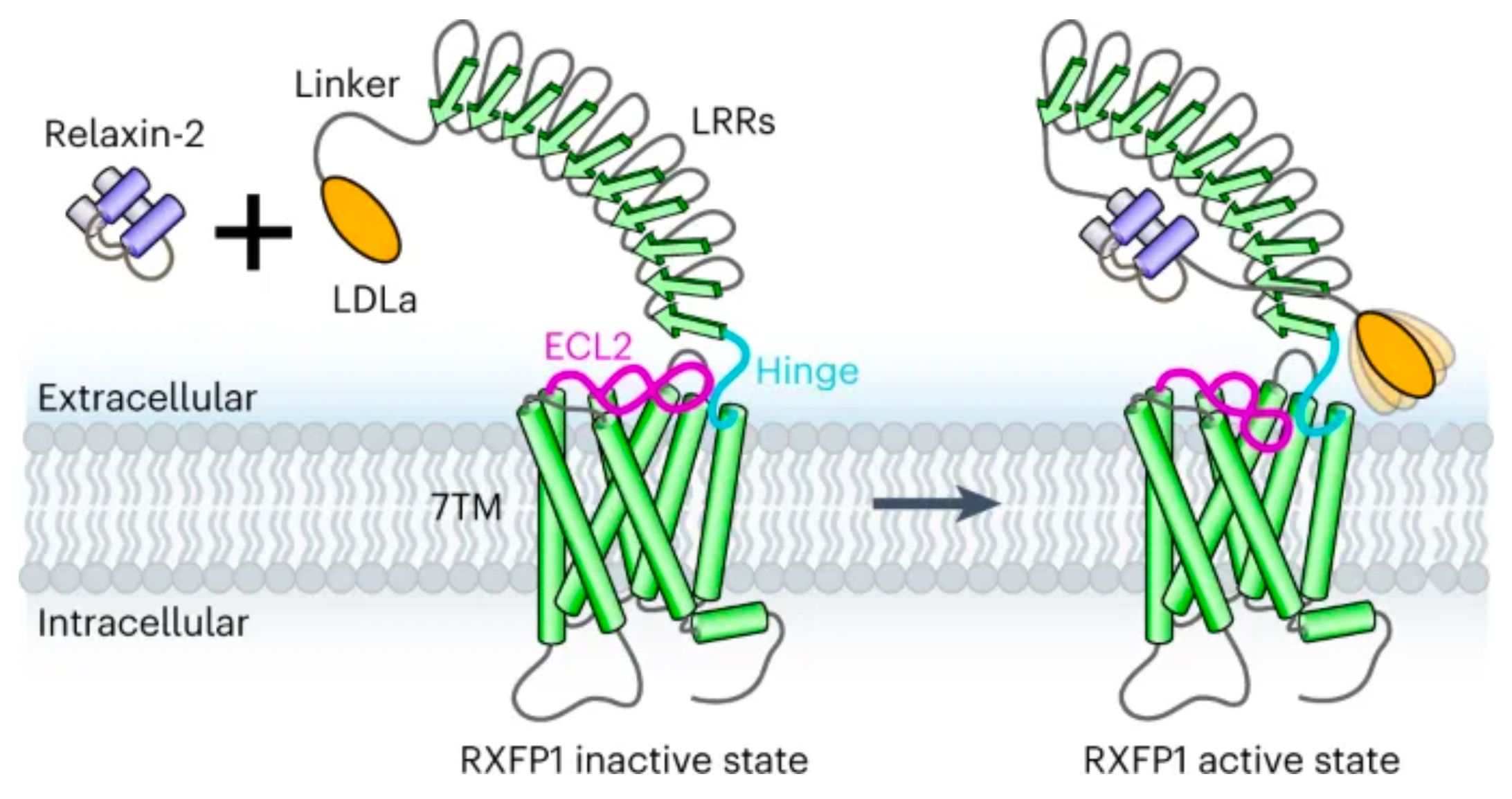
On April 20th, 2023, the team led by Andrew Kruse at Harvard University published a new mechanism by which the relaxin receptor RXFP1 transmits regulatory signals through an autoregulatory mechanism in the online edition of the journal Nature Chemical Biology.

In the study, Professor Andrew C. Kruse's laboratory resolved the cryo-electron microscopy structure of the RXFP1 receptor in complex with the downstream remodeled miniGs protein trimer. The structure revealed that the extracellular loop 2 (ECL2) of RXFP1 occupies the GPCR orthogonal ligand-binding pocket in its active state. Subsequent structural and functional studies have determined that the conformation of ECL2 is regulated by a mechanism involving the receptor's leucine-rich repeats (LRRs) and the hinge region (a short segment between LRRs and the 7-transmembrane domains, or 7TMs). These studies suggest that the synergistic action of multiple receptor structural domains controls the transduction of RXFP1 signaling under the influence of its agonist, relaxin-2.
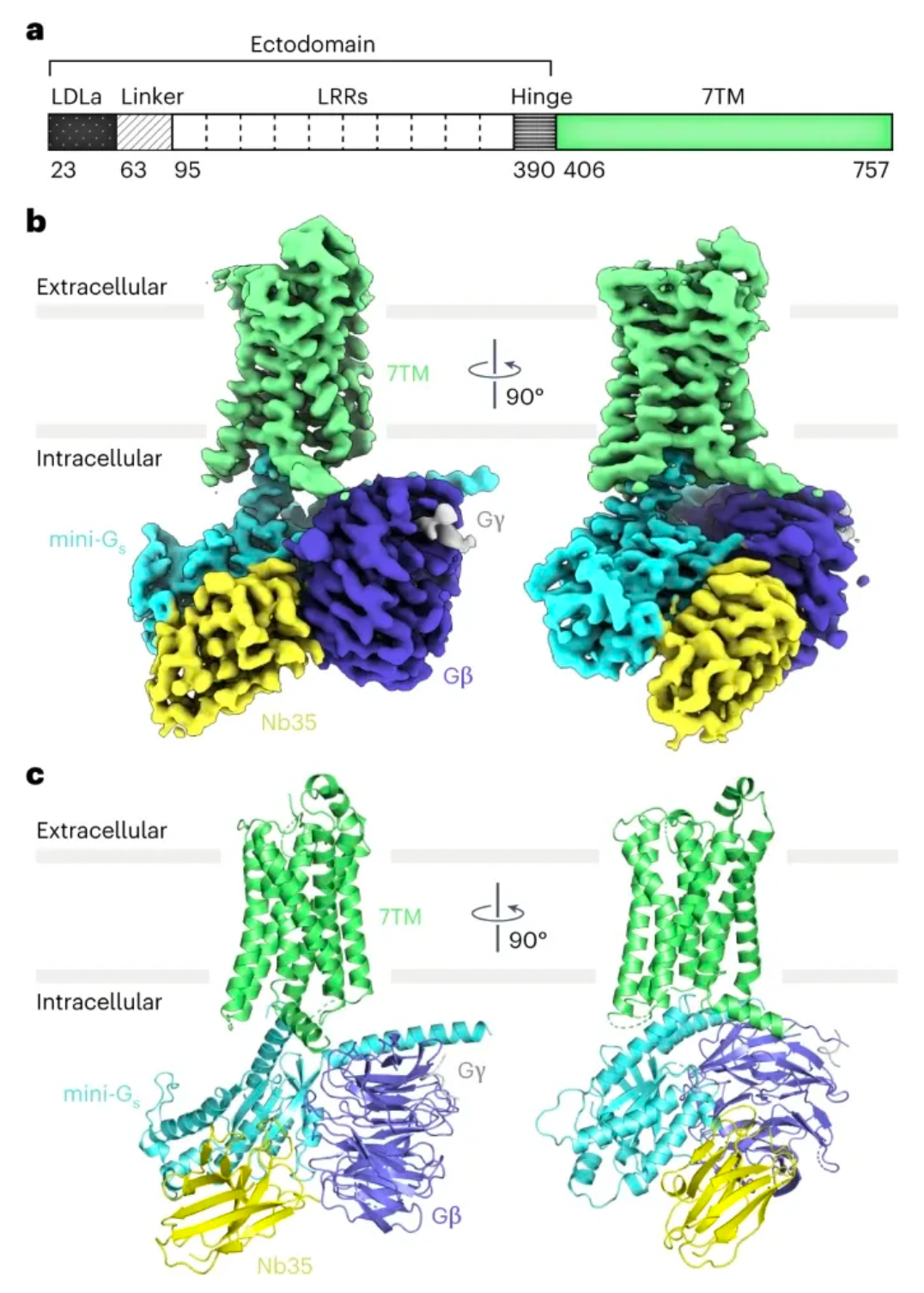
Combining mutagenesis experiments and studies on second messenger activity at the cellular level, the authors validated the molecular mechanism by which ECL2 regulates receptor activation. Integrating multidimensional experimental validations such as molecular dynamics simulations, cryo-electron microscopy, and crosslinking mass spectrometry, the authors proposed a new model for the auto-inhibition regulation mechanism of the relaxin receptor RXFP1.
Drug Development Targeting the Relaxin Receptor
According to statistics from the Synapse Database, there are a total of 11 new drug development pipelines worldwide focusing on relaxin receptors. Among them, recombinant relaxin-2 Serelaxin was approved in Russia in 2015 for the treatment of fibrosis, heart failure, and portal hypertension.
Serelaxin (RLX030, Novartis) is a recombinant form of human relaxin-2, a naturally occurring peptide. It is a hormone produced during pregnancy, which softens the cervix and the pubic symphysis (the joint at the pubic bone) and facilitates the birth process by allowing these areas to expand. It is a heterodimeric protein secreted by the corpus luteum and placenta during pregnancy.
According to a clinical study published in The Lancet in 2013, treatment with Serelaxin for acute heart failure can relieve dyspnea and improve other clinical outcomes, but it does not affect readmission for treatment. Additionally, the tolerance and safety of Serelaxin treatment are good.

In the U.S. market, Serelaxin (RLX030), developed by Novartis, was granted the designation of a "Breakthrough Therapy" by the FDA in 2013. However, in 2014, both the U.S. FDA and European regulatory authorities rejected the marketing application for RLX030, stating that the initial, smaller scale Phase III RELAX-AHF study did not prove the drug's ability to provide short-term relief of dyspnea within 24 hours, and further research was required to demonstrate its efficacy. In 2017, a global trial involving 6,600 patients over a period of 3.5 years, the RELAX-AHF-2 trial, evaluated the treatment effects of Serelaxin in addition to standard therapy, but it failed to meet its primary endpoints of reducing cardiovascular death by day 180, or worsening heart failure by day 5. Novartis claims that Serelaxin is a recombinant form of human relaxin-2 hormone, which is produced at higher levels in pregnant women, and the company believes it could help meet the increased demands on the cardiovascular system during pregnancy.

The recombinant polypeptide AZD-3427 and the small molecule compound AZD-5462, developed by AstraZeneca, are currently in Phase 2 and Phase 1 clinical trials, respectively.
Recently, AstraZeneca has initiated patient enrollment for the Phase 2b clinical trial of AZD-3427. The indications for this trial are heart failure and pulmonary arterial hypertension caused by left heart disease.