REGN7075 Bispecific Antibody Shows Anti-Tumor Effects with Libtayo, Findings at ASCO
Regeneron Pharmaceuticals, Inc. released positive outcomes from an ongoing Phase 1/2 clinical study assessing its pioneering costimulatory bispecific antibody, REGN7075 (EGFRxCD28), combined with Libtayo® (cemiplimab) in patients suffering from advanced solid tumors. Findings from the dose-escalation phase demonstrated that this investigational combination elicited anti-tumor responses in patients with microsatellite stable colorectal cancer.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
REGN7075 is pioneering among immunotherapies in demonstrating efficacy in MSS CRC, notably in a patient with hepatic metastases. The findings are set to be presented during an oral session at the American Society of Clinical Oncology 2024 Annual Meeting in Chicago.
"Microsatellite stable colorectal cancer has traditionally shown resistance to immunotherapy," said Neil H. Segal, M.D., Ph.D., a Medical Oncologist and Research Director in the Division of Gastrointestinal Oncology at Memorial Sloan Kettering Cancer Center, and a trial investigator. "Preliminary data for this innovative EGFRxCD28 costimulatory bispecific when combined with Libtayo are promising, indicating anti-tumor activity in a particularly hard-to-treat cancer. This therapeutic regime is among the first immunotherapies to exhibit clinical activity in microsatellite stable colorectal cancer, and we are eager to extend this trial to additional cancer types."
In the dose-escalation phase of the study, patients with metastatic or locally advanced solid tumors – who had no remaining standard treatment options, most of whom also had liver metastases – received a combination therapy of REGN7075 and Libtayo, preceded by a lead-in dose of REGN7075 monotherapy.
"Regeneron is dedicated to developing an innovative oncology portfolio that includes checkpoint inhibitors, CD3 bispecifics, and CD28 costimulatory bispecifics. Over recent years, we have advanced our programs in checkpoint inhibitors and the CD3 category, and we are now observing promising activity with two costimulatory bispecific antibodies," stated Israel Lowy, M.D., Ph.D., Senior Vice President, Translational and Clinical Oncology at Regeneron.
The combination of REGN7075 and Libtayo is in active clinical development, and its safety and efficacy have not yet been fully assessed by any regulatory authority. While the dose-escalation phase for multiple solid tumor types, including non-small cell lung cancer, colorectal cancer, head and neck cancer, and others is progressing, expansion cohorts in several tumor types have also commenced.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
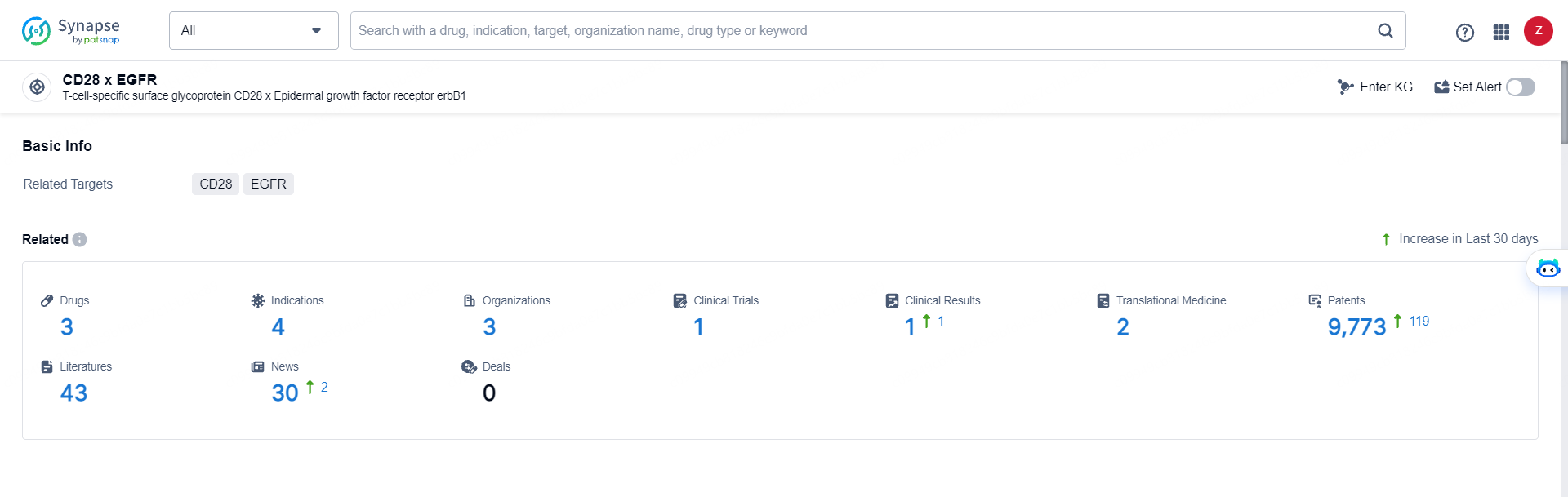
According to the data provided by the Synapse Database, As of May 29, 2024, there are 3 investigational drugs for the CD28 x EGFR targets, including 4 indications, 3 R&D institutions involved, with related clinical trials reaching 1, and as many as 9773 patents.
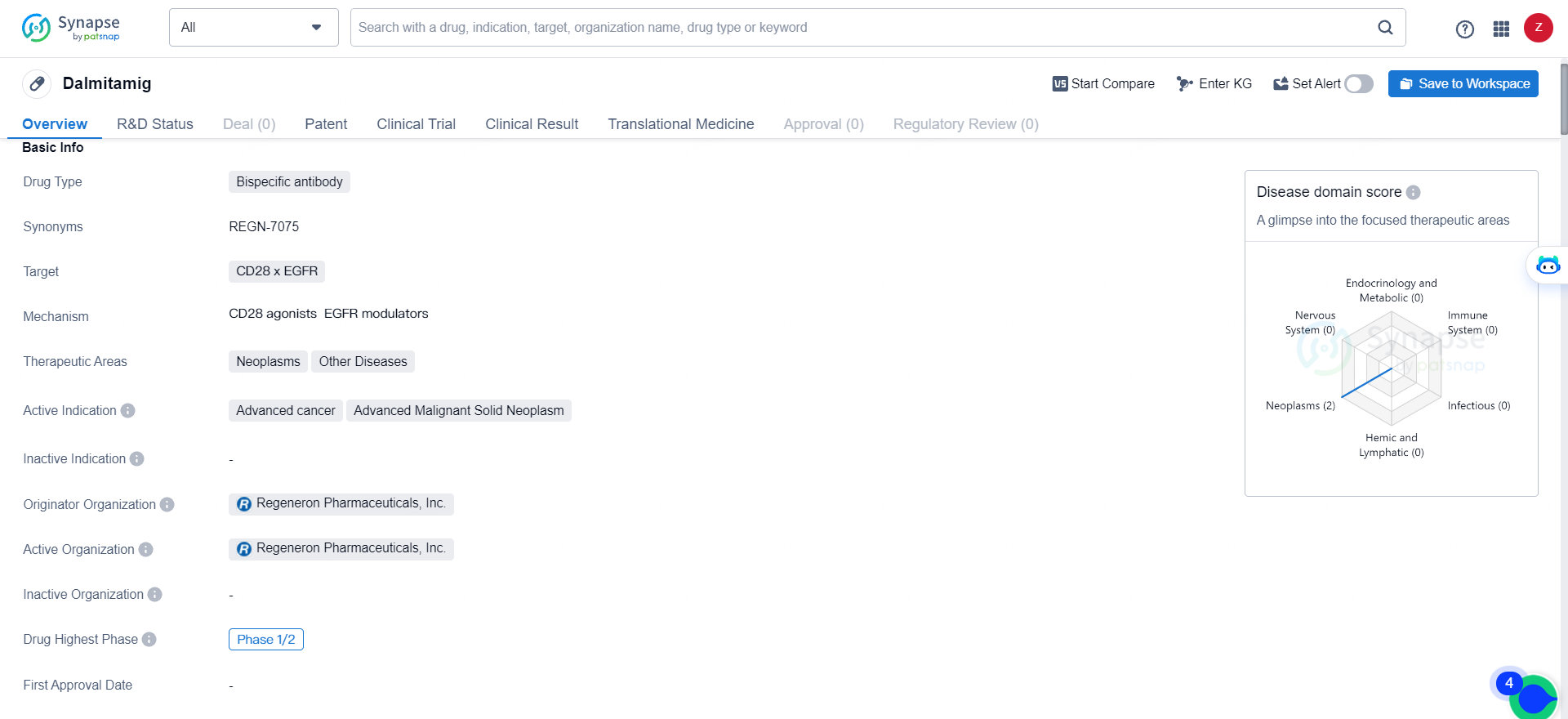
Dalmitamig is a bispecific antibody drug targeting CD28 x EGFR, with a focus on treating advanced cancer and advanced malignant solid neoplasms. Its development by Regeneron Pharmaceuticals, Inc. and its current Phase 1/2 status indicate its potential as a promising therapeutic option for patients with these conditions.