VISEN Announces China's Approval to Submit Lonapegsomatropin Biologic License
VISEN Pharmaceuticals, a pioneering biotherapeutics enterprise with a concentration on hormonal disorders, declared that the China National Medical Products Administration has given the green light to the company's submission of a Biologics License Application for Lonapegsomatropin.
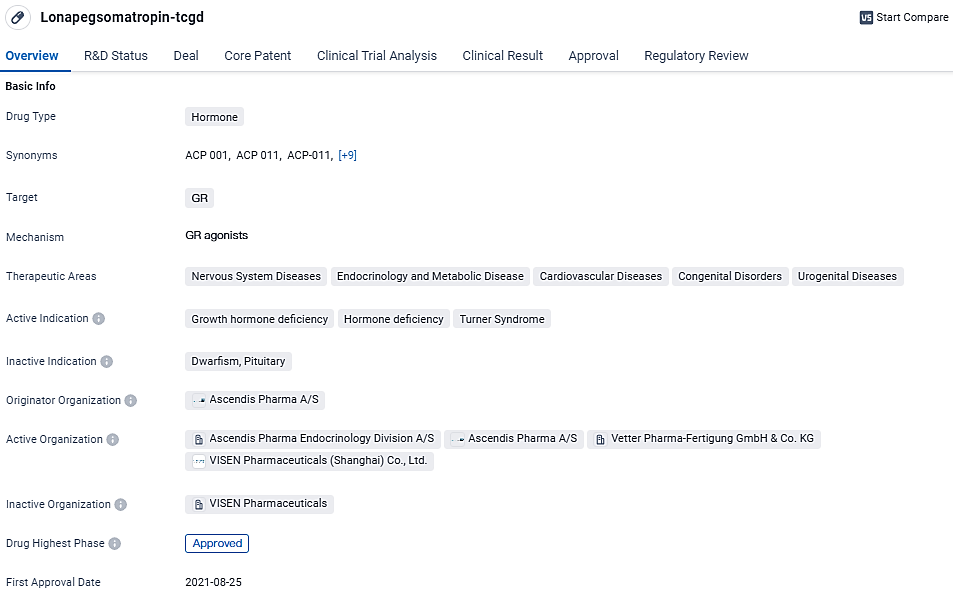
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The groundbreaking growth hormone known as Lonapegsomatropin has received approval for use on a once-weekly basis for the management of Pediatric Growth Hormone Deficiency, with the endorsement coming from both the U.S. Food & Drug Administration and the European Medicines Agency.
VISEN Pharmaceuticals' Chief Executive Officer, Mr. Pony Lu, remarked, "Receiving the Biologics License Application approval is a pivotal moment in the journey towards bringing this product to the market. We are committed to focusing on the delivery of top-tier or pioneering medications, aiming to enhance the therapy available to Chinese patients suffering from endocrine disorders, which in turn should lead to improved management and health outcomes."
As a precursor to somatropin, Lonapegsomatropin is introduced into the body on a weekly basis. With the assistance of the cutting-edge TransConTM technology, this therapy has been tailored to ensure the consistent release of somatropin in its pure form - a therapy aligning with the somatropin currently employed in day-to-day treatments of pediatric GHD. The somatropin released from Lonapegsomatropin maintains its natural state, mirroring the 191 amino-acid make-up and dimensions of the body's own growth hormone.
Ownership and development of Lonapegsomatropin and the transformative TransCon technology belong to Ascendis Pharma A/S. Meanwhile, VISEN Pharmaceuticals possesses the exclusive authorization to further develop and market Lonapegsomatropin across the extensive Chinese market.
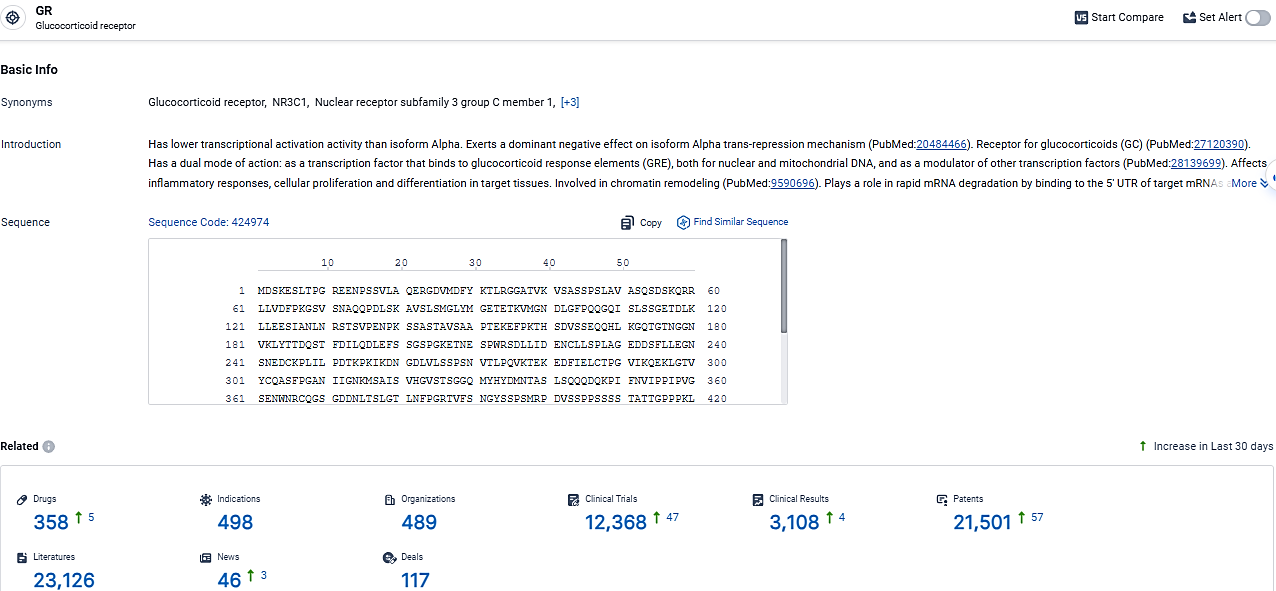
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 11, 2024, there are 358 investigational drugs for the GR target, including 498 indications, 489 R&D institutions involved, with related clinical trials reaching 12368, and as many as 21501 patents.
Lonapegsomatropin-tcgd targets the GR and is indicated for growth hormone deficiency, hormone deficiency, and Turner Syndrome. With its recent approval in the United States and orphan drug status, Lonapegsomatropin-tcgd holds promise in addressing various diseases within the nervous system, endocrinology, cardiovascular, congenital, and urogenital domains.