Relay Therapeutics Shares Interim Results for RLY-2608 Showing Significant Progression-Free Survival
Relay Therapeutics, Inc. (Nasdaq: RLAY), a company focused on precision medicine and revolutionizing drug discovery through advanced computational and experimental approaches, has announced encouraging interim results for RLY-2608, the initial investigational allosteric, pan-mutant, and isoform-selective inhibitor of PI3Kα. The interim data indicated that patients with PI3Kα-mutated, HR+, HER2- locally advanced or metastatic breast cancer, who were heavily pre-treated and received RLY-2608 600mg BID combined with fulvestrant, exhibited clinically significant progression free survival (PFS).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Dr. Don Bergstrom, M.D., Ph.D., President of R&D at Relay Therapeutics, highlighted that preliminary results indicate RLY-2608 could potentially provide unprecedented benefits to patients by specifically targeting mutant PI3Kα, with considerably reduced toxicity compared to current non-selective treatments. “We’re very pleased to observe that combining RLY-2608 with fulvestrant has resulted in significant progression-free survival in patients with heavily pre-treated PI3Kα-mutated, HR+, HER2- metastatic breast cancer. We aim to promptly communicate these findings to regulatory bodies and finalize the design of a pivotal trial, which we plan to launch in 2025.”
Currently, RLY-2608 is under investigation in the ReDiscover study, a first-of-its-kind human trial designed to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and initial antitumor activity of RLY-2608 alone, in combination with fulvestrant, and coupled with either ribociclib or atirmociclib (Pfizer’s selective CDK4 inhibitor).
By the interim data cut-off on August 12, 2024, the study's RLY-2608 + fulvestrant arm had enrolled 118 patients with PI3Kα-mutated, HR+, HER2- locally advanced or metastatic breast cancer, covering all dosages in both dose escalation and expansion phases. This cohort included 64 patients at the recommended Phase 2 dose (RP2D) of 600mg BID (17 in dose escalation and 47 in dose expansion). Among these 64, 31 patients had a kinase mutation while 33 had a non-kinase mutation. Twelve patients harboring a PTEN or AKT co-mutation were excluded from the efficacy analysis, aligning with the currently proposed pivotal population. An abstract detailing these findings has been submitted for presentation at the San Antonio Breast Cancer Symposium, scheduled for December 10-13, 2024.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
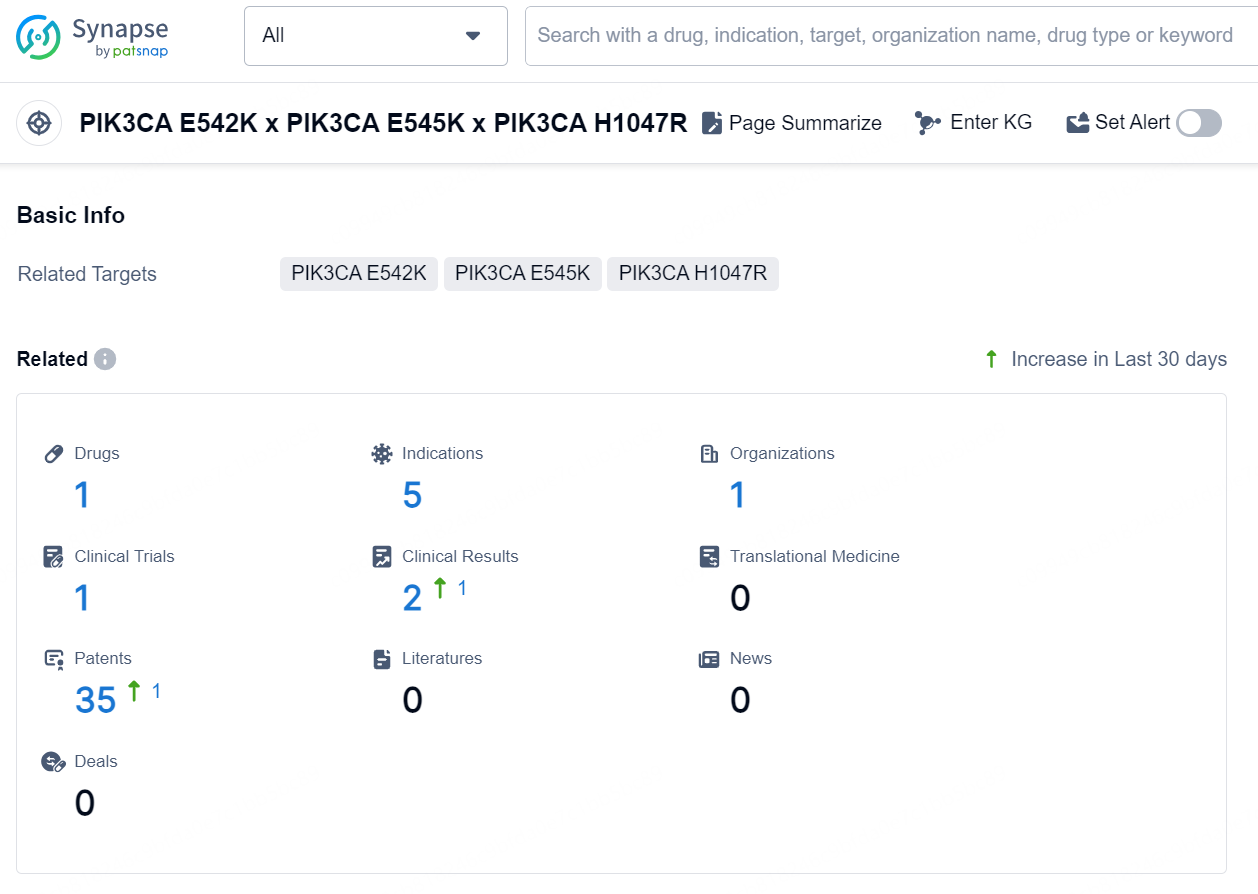
According to the data provided by the Synapse Database, As of September 11, 2024, there are 1 investigational drug for the PIK3CA E542K x PIK3CA E545K x PIK3CA H1047R targets, including 5 indications, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 35 patents.
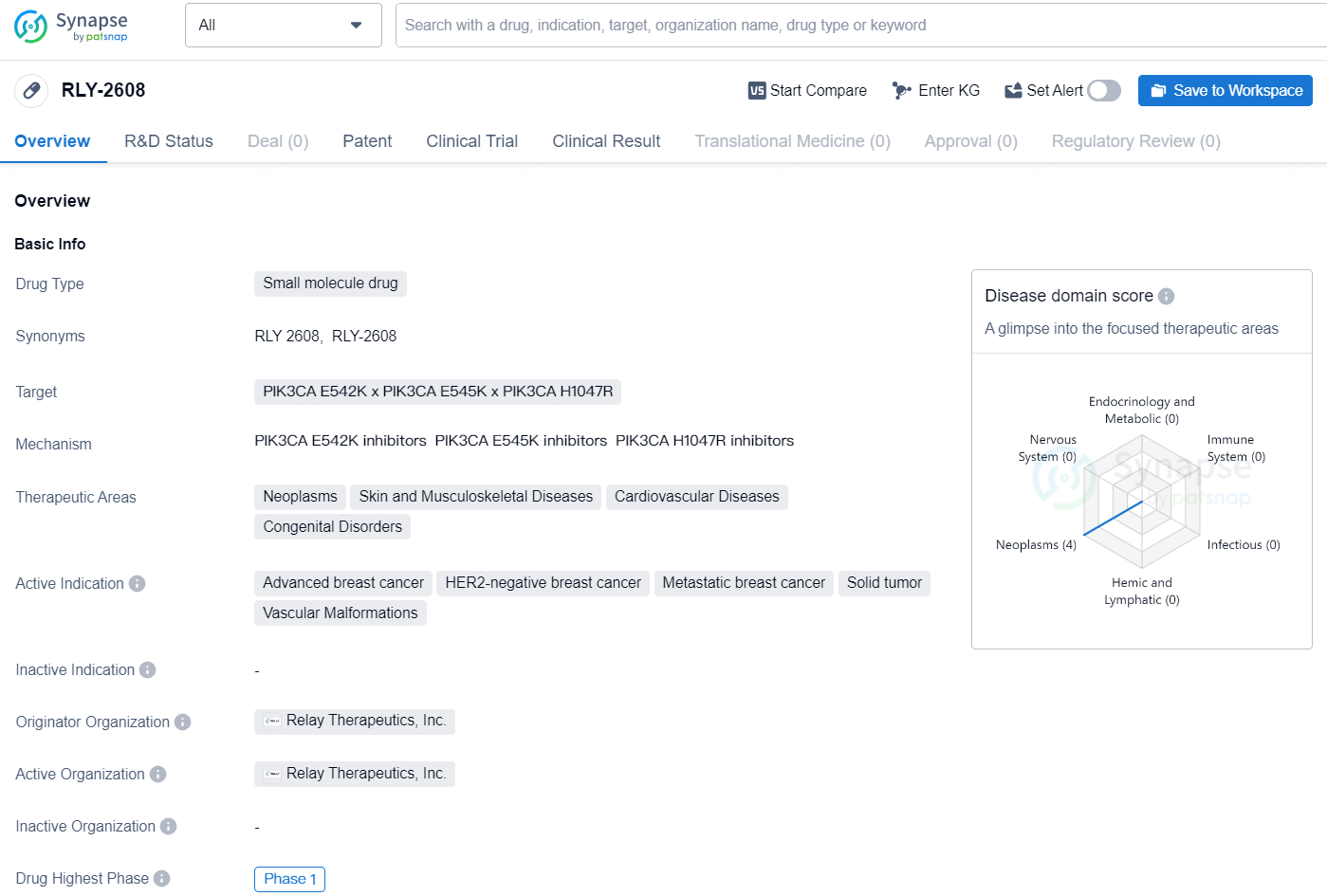
The drug RLY-2608 is a small molecule drug developed by Relay Therapeutics, Inc. It targets specific mutations in the PIK3CA gene, including E542K, E545K, and H1047R. These genetic mutations are associated with various therapeutic areas, including neoplasms, skin and musculoskeletal diseases, cardiovascular diseases, and congenital disorders. The drug is being developed for the treatment of advanced breast cancer, HER2-negative breast cancer, metastatic breast cancer, solid tumors, and vascular malformations.