Ruxolitinib Phosphate: A Quick Look at Its R&D Progress and Clinical Results from the 2023 ASH
On 10 Dec 2023, the 3-Year final analysis of efficacy and safety of Ruxolitinib from the phase III REACH3 study will be unveiled at the 2023 ASH Congress.
Ruxolitinib Phosphate's R&D Progress
Ruxolitinib Phosphate is a small molecule drug that targets JAK1 and JAK2, making it suitable for the treatment of various diseases. It has been approved for use in several therapeutic areas, including Hemic and Lymphatic Diseases, Neoplasms, Immune System Diseases, Skin and Musculoskeletal Diseases, Respiratory Diseases, Other Diseases, and Congenital Disorders. 
According to the Patsnap Synapse, Ruxolitinib Phosphate has received approval in multiple countries, with the first approval granted in the United States in November 2011. And the clinical trial area for Ruxolitinib Phosphate is primarily in the United States, China and United Kingdom. The key indication is Primary Myelofibrosis. 
Detailed Clinical Result of Ruxolitinib Phosphate
This randomized, parallel assignment, open Labeled study (NCT03112603) was conducted in in patients With chronic graft-versus-host disease.

In this study, pts ≥12 years of age with moderate or severe SR/D-cGVHD were randomized 1:1 to receive either RUX 10 mg twice daily (BID) or investigator-selected BAT and followed for 3 years, till discontinuation or death. The primary analysis was conducted at week 24 (Cycle 7 Day 1 [C7D1]) in randomized pts, then pts entered the extension period (C7–39) in which they continued treatment, switched from BAT to RUX (crossover cohort), or discontinued treatment and entered long-term survival follow-up (FU). Failure-free survival (FFS; key secondary endpoint), duration of response (DOR), overall survival (OS), non-relapse mortality (NRM), malignancy relapse (MR), and safety were analyzed at 3 years (week 156; final data cut off 15 Dec 2022). Overall response rate (ORR) and best overall response (BOR) during the crossover treatment period were analyzed for pts who switched from BAT to RUX on or after C7D1.
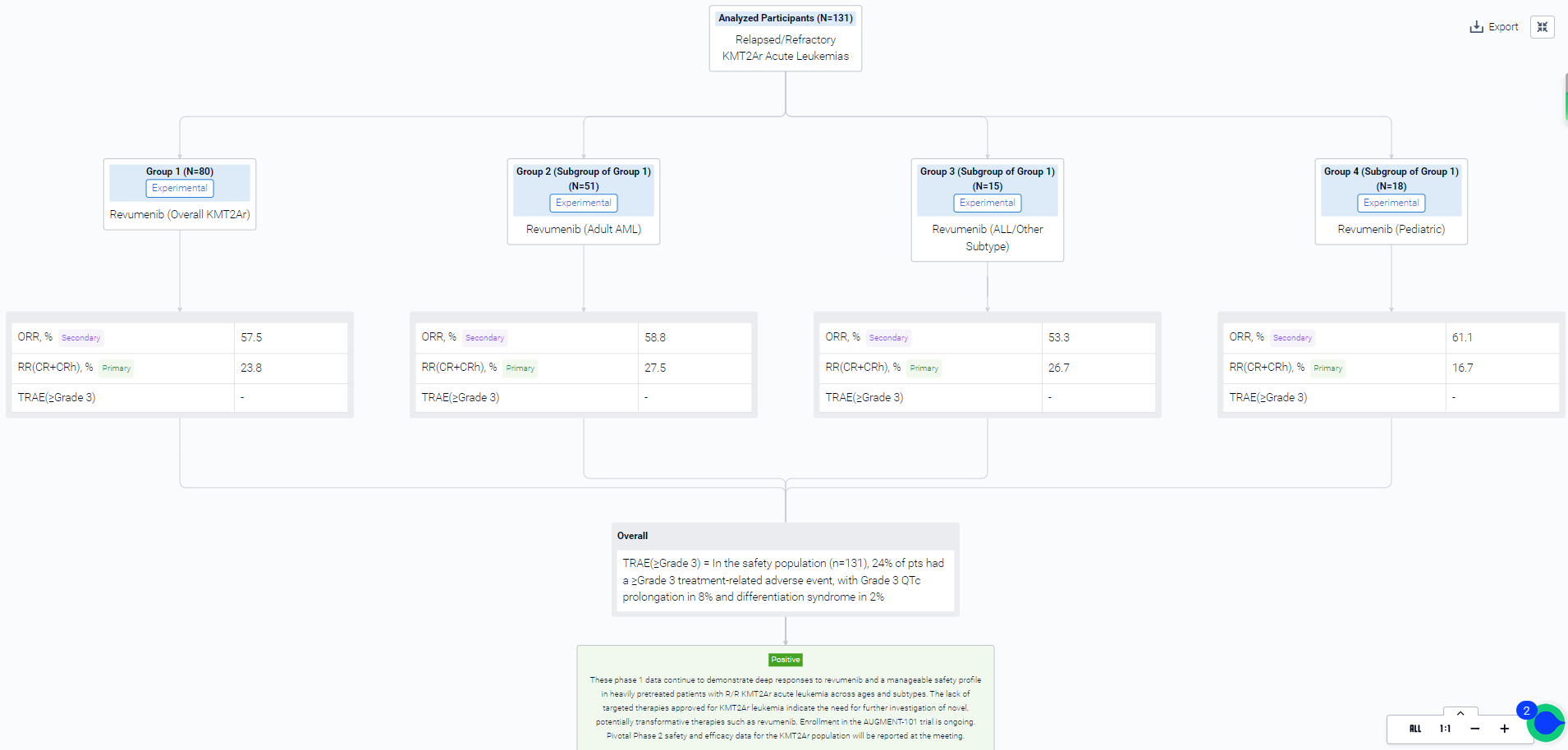
The result showed that of 329 pts randomized, 53 completed the treatment period and 276 discontinued, 115 entered survival FU (RUX: 73/165; BAT:42/164); 70 pts crossed over from BAT to RUX of which 16 completed the crossover treatment period and 24 entered survival FU. Median FFS was longer in the RUX vs BAT arms (38.4 vs 5.7 months; hazard ratio [HR]=0.361, 95% confidence interval [CI]: 0.268, 0.485) with 12-month FFS probabilities of 64.0% (95% CI: 56.1, 70.8) and 28.8% (95% CI: 21.8, 36.1), respectively. Median OS was not reached and there was no difference in risk of death between the arms (HR=0.851, 95% CI: 0.544, 1.331) . Median DOR was 6.4 months (95% CI: 4.9, 11.4) in the BAT arm but was not reached for the RUX arm; notably, the probability of maintaining DOR at 3 years was higher in RUX (59.6%; 95% CI: 50.4, 67.6) vs BAT (26.7%; 95% CI: 18.5, 35.5). NRM event rates were similar between the arms (RUX: 29/165; BAT 34/164), and MR events were similar and low in both arms (13/156 and 11/160, RUX and BAT respectively) up to 3 years. The ORR at week 24 after crossover from BAT to RUX was 50.0% (95% CI: 37.8, 62.2), including complete response (CR) in 4 (5.7%) pts and partial response (PR) in 31 (44.3%) pts. The BOR during crossover was 81.4% (95% CI: 70.3, 89.7), including CR of 7.1% and PR of 74.3%; disease progression only occurred in 1 pt. Almost all pts treated during the main treatment period (RUX: 100%; BAT: 93.7%) experienced ≥1 adverse event (AE) and, in general, AE rates were higher in the RUX vs BAT arms, likely due to prolonged FU and exposure to RUX (median exposure to treatment of 52.9 weeks vs. 24.1 weeks for RUX and BAT, respectively). Anemia was the most common AE (RUX: 33.9%; BAT: 15.8%) and grade ≥3 AE for RUX (17.6% vs BAT 9.5%, respectively); grade ≥3 neutropenia, thrombocytopenia, alanine aminotransferase increase, and gamma-glutamyltransferase increase were also ≥5% higher for RUX than BAT. Anemia (24.2%; grade ≥3, 10.3%) and thrombocytopenia (3.8%) were the most common RUX and BAT treatment-related AEs, respectively. Infections, excluding tuberculosis, were the most common AE of special interest (77.6% vs. 68.4%; grade ≥3, 31.5% and 26.6%, for RUX and BAT, respectively). On-treatment deaths were mainly due to cGvHD (n/N, RUX: 10/18; BAT 6/12).
It can be concluded that after 3 years of treatment in REACH3, the longer FFS and higher DOR with RUX vs BAT indicated that cGVHD was more in control with RUX treatment. Notably, efficacy was demonstrated in pts who switched from BAT to RUX with an ORR similar to that in pts randomized to RUX. RUX was well tolerated with no unexpected toxicities and safety that was consistent with earlier studies. Overall, the long-term control of cGVHD and tolerability of RUX was demonstrated for pts ≥12 years of age with SR/D-cGvHD.
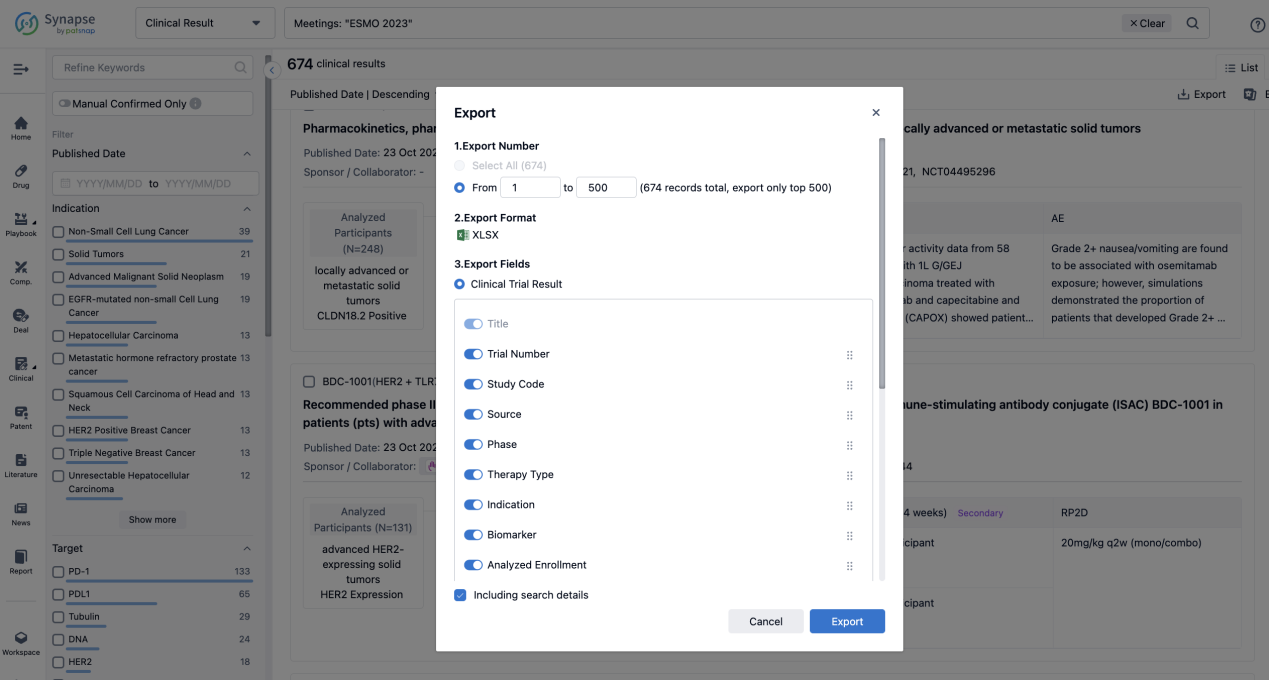
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.

Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!