Sana Biotech's SC291, an off-the-shelf CAR T targeting CD19, undetected by immune system in initial clinical results
Sana Biotechnology, Inc., a firm dedicated to redefining patient treatment possibilities using modified cellular therapies, has revealed the publication of a summary in the journal Blood. This summary contains the initial clinical results obtained from the inaugural participant treated with the minimal dosage during the active ARDENT Phase 1 study. The treatment being investigated is SC291, a CAR T cell therapy with hypoimmune modifications aimed at targeting CD19 for allogeneic use.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
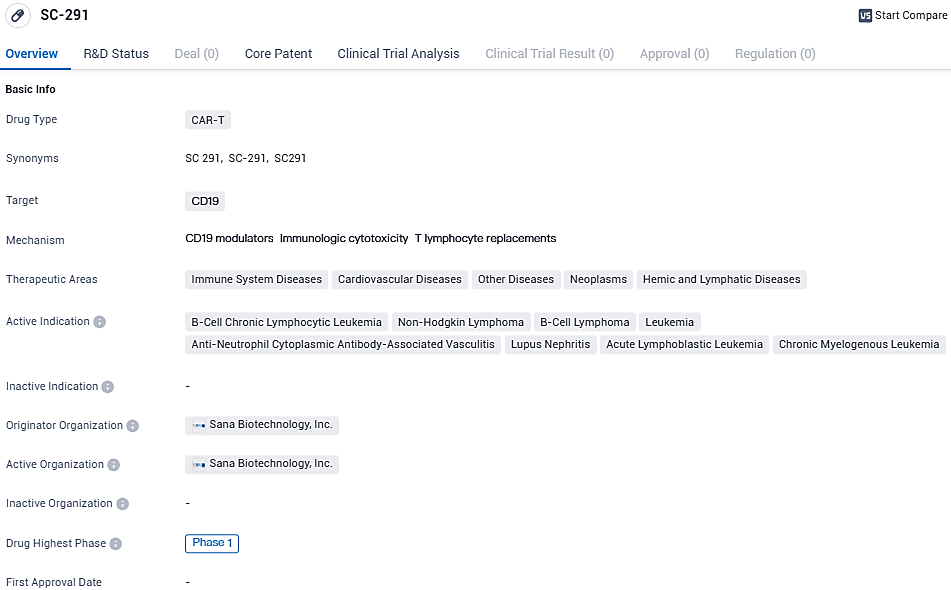
In the clinical setting, SC291 has shown a favorable safety profile and been well accepted, successfully avoiding detection by the immune system and achieving a measure of disease remission in a case of chronic lymphocytic leukemia. The ARDENT trial, a preliminary Phase 1 investigation, is currently assessing the safety and tolerability of SC291 for individuals afflicted with CLL and non-Hodgkin lymphoma. As the dose-escalation phase of the study progresses, the involved corporation is preparing to share additional results in a future scientific assembly.
Gary Meininger, MD, the Chief Medical Officer at Sana, reported, "These inaugural clinical findings reveal that our HIP technology is capable of modifying allogeneic cells in such a way that they become invisible to both the adaptive and innate immune responses, even when faced with a fully functioning immune system. This addresses a significant hurdle in harnessing the therapeutic potential of allogeneic cells."
Dr. Meininger further commented on the implications of the research, stating, "These preliminary results hint at SC291's ability to both endure within the body and target malignant cells in a fashion akin to autologous cells. This prospect, along with the scalability of our production process, gives us reason to be optimistic about SC291 and our suite of HIP-tweaked cellular therapies contributing meaningful treatment options for patients." These observations have been shared in a synopsis submitted previously in the year and more details from this active clinical research are anticipated, which will shed extra light on the therapeutic characteristics of SC291.
The investigational therapy SC291 is a hypoimmune, CD19-specific allogeneic CAR T cell therapy constructed from genetically modified CD4+ and CD8+ T cells procured from healthy donors. Utilizing Sana's hypoimmune platform aimed at precluding immune rejection of foreign cells, SC291 has the potential to enhance CAR T cell survival and may lead to an augmented rate of sustainable, complete remissions in patients battling various B-cell lymphomas or leukemias.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
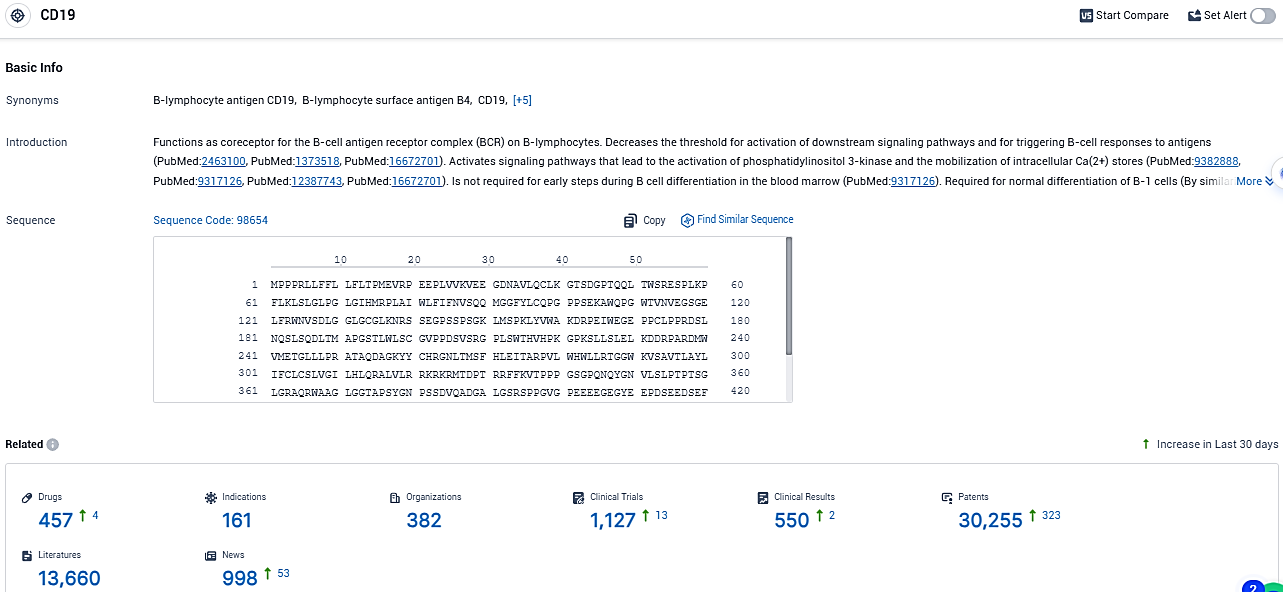
According to the data provided by the Synapse Database, As of December 12, 2023, there are 457 investigational drugs for the CD19 target, including 161 indications, 382 R&D institutions involved, with related clinical trials reaching 1127, and as many as 30255 patents.
SC-291 targets CD19 and has potential applications in immune system diseases, cardiovascular diseases, other diseases, neoplasms, and hemic and lymphatic diseases. The drug is currently in Phase 1 of clinical development, and further research is needed to determine its safety and efficacy in treating the indicated conditions.