Satellos Reports Promising SAT-3247 Results for Muscle Recovery in Duchenne Muscular Dystrophy Dog Model
Satellos Bioscience Inc., a publicly traded biotechnology firm engaged in creating novel small molecule treatments aimed at enhancing therapies for muscle diseases and disorders, has disclosed initial findings from a single case study involving SAT-3247 treatment in a dog model of Duchenne muscular dystrophy.
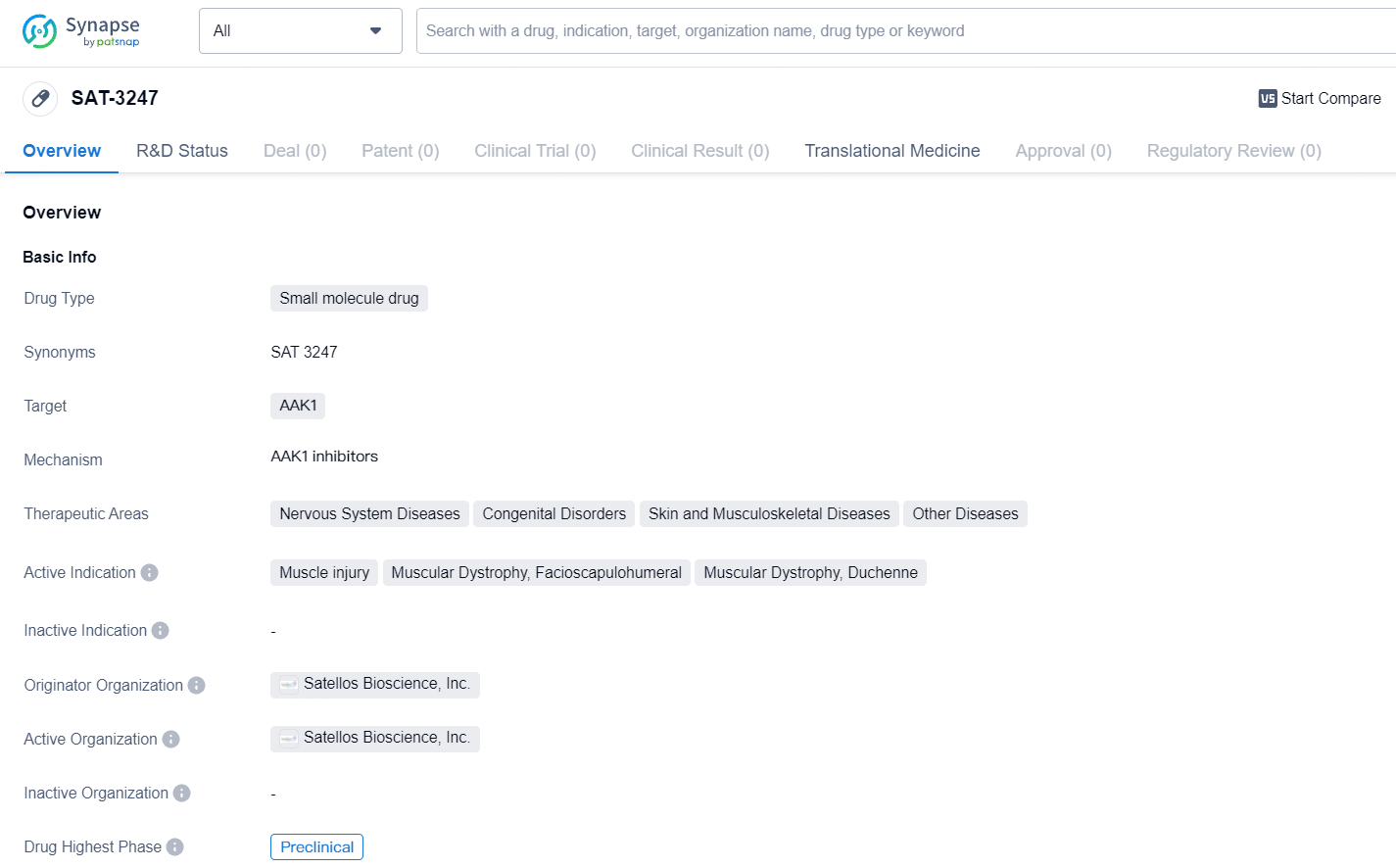
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This model exemplifies a severe dystrophy that mirrors the disease progression observed in individuals with DMD. SAT-3247 is being developed as an innovative regenerative medicine strategy for treating DMD. These findings were presented at the Parent Project Muscular Dystrophy 30th Annual Conference over the past weekend.
"We are heartened by the initial data demonstrating that SAT-3247 treatment enhanced regeneration and muscle strength in a canine model of Duchenne," stated Frank Gleeson, Co-founder and CEO of Satellos Bioscience.
"As Satellos' inaugural results in a large animal study, we are pleased that these outcomes continue to substantiate the improvements in muscle repair and regeneration we've consistently observed in the mdx mouse model. This preliminary study further endorses the potential of SAT-3247 treatment to restore muscle repair and regeneration in individuals with Duchenne," added Frank Gleeson.
Post-treatment with SAT-3247, the animals displayed an increase in Regenerative Index, which measures the number of newly regenerated muscle fibers relative to damaged and dying muscle fibers.
"Increases in the Regenerative Index indicate that muscle repair and regeneration are taking place," commented Phil Lambert, Ph.D., Chief Scientific Officer of Satellos Bioscience. "Although preliminary, these results support our understanding of SAT-3247's unique mechanism of action. We are working diligently to progress this novel small molecule drug candidate into a first-in-human clinical trial this quarter."
SAT-3247 is designed as a once-daily oral small molecule drug that addresses the root cause of muscle loss in degenerative diseases, particularly Duchenne. SAT-3247 introduces a novel mechanism of action to restore impaired muscle regeneration resulting from the lack of functional dystrophin.
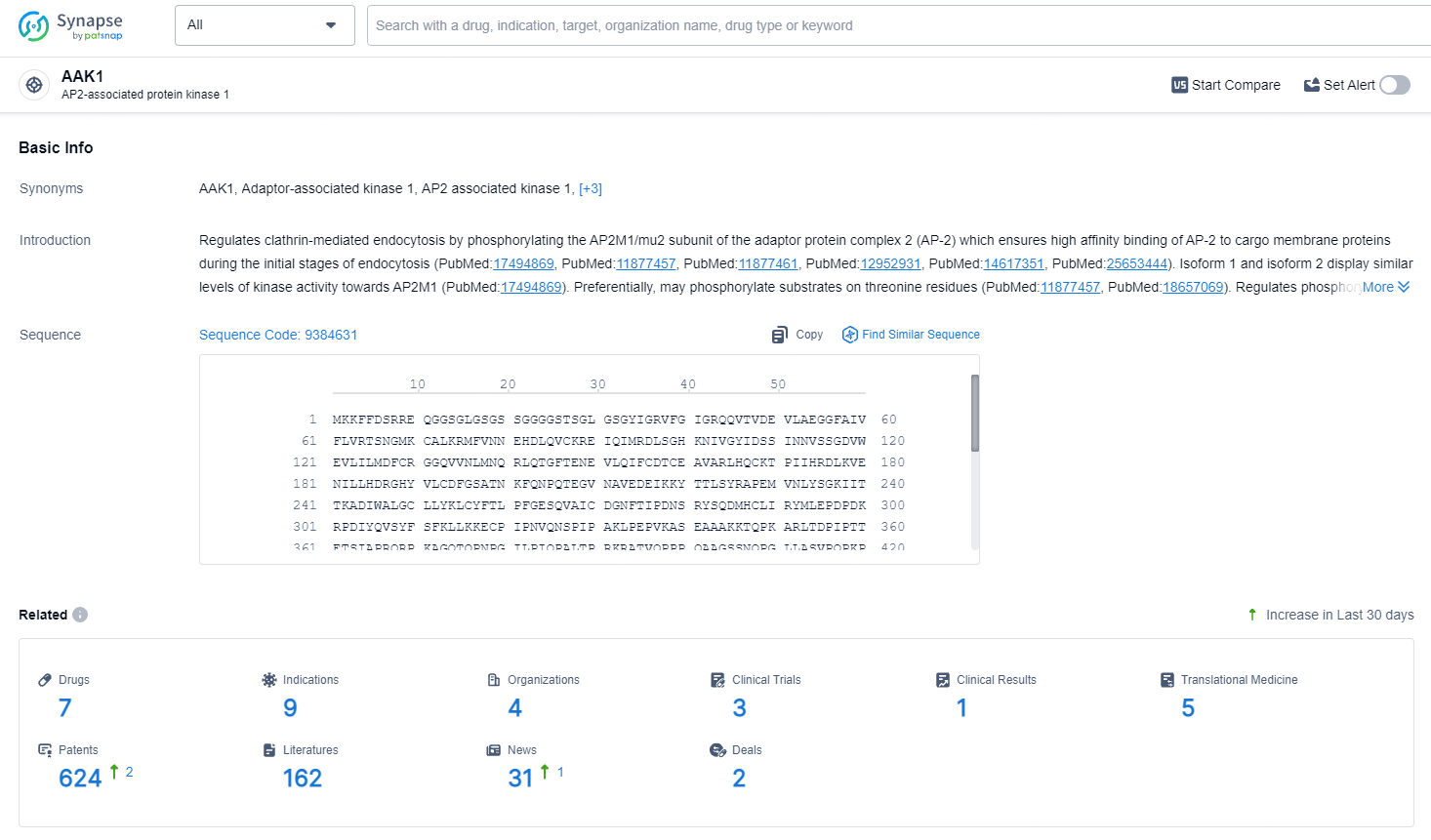
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 8, 2024, there are 7 investigational drugs for the AAK1 target, including 9 indications, 4 R&D institutions involved, with related clinical trials reaching 3, and as many as 624 patents.
SAT-3247 targets AAK1 and intended for the treatment of various diseases, with a focus on muscle-related conditions. While the drug is in the preclinical phase, further research and development efforts will be needed to assess its potential for clinical use and eventual market availability.