SGLT2 Inhibitors - The Cross-Domain Superstar of Antidiabetic Drugs
Diabetes is a global public health challenge that all of us face together. Based on unmet clinical needs, research and development of innovative drugs in this field has never ceased.
Glucose is filtered through the renal glomeruli and reabsorbed in the proximal tubules. In the body, glucose cannot freely pass through the lipid bilayer of the cell membrane and must rely on glucose transport proteins on the cell membrane. SGLT2 (sodium-glucose co-transporter 2) plays a major role in the reabsorption of glucose, and SGLT2 inhibitors have been the rising stars in the field of glucose-lowering drugs in recent years. According to their mechanism of action, SGLT2 inhibitors primarily lower blood glucose levels by inhibiting renal tubular SGLT2, lowering the renal glucose threshold, reducing the reabsorption of glucose in the renal tubules, and increasing the excretion of glucose in the urine.
SGLT2 inhibitors use a unique insulin-independent mechanism to lower blood glucose levels, not only effectively and safely reducing glucose, but also providing significant cardio-renal protection. Their role in diabetes therapy has been elevated, and they have also been unanimously recognized by consensus among experts in the field of heart failure and Chronic Kidney Disease (CKD). They have been acclaimed as "cross-border stars" in the world of glucose-lowering drugs, and it is highly anticipated that they will become legendary classics in the history of world medicine, alongside penicillin, aspirin, and diazepam.
SGLT2 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 2 Sep 2023, there are a total of 82 SGLT2 drugs worldwide, from 116 organizations, covering 33 indications, and conducting 2075 clinical trials. The analysis of the target SGLT2 reveals a competitive landscape with multiple companies actively involved in the development of drugs. C.H. Boehringer Sohn AG & Co. KG, AstraZeneca PLC, Merck & Co., Inc., and Johnson & Johnson are the companies growing fastest under the current target. These companies have a significant number of drugs in various stages of development, indicating their commitment to R&D.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
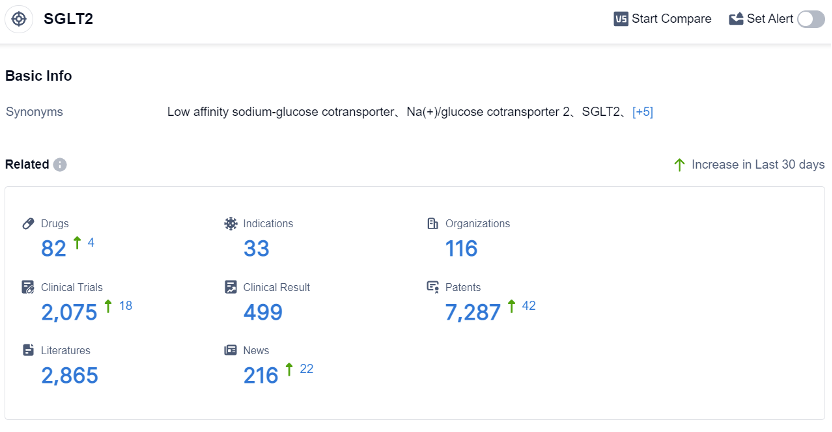
The most common approved indication for drugs targeting SGLT2 is Diabetes Mellitus, Type 2. This indicates the focus of the pharmaceutical industry on addressing this medical condition. Small molecule drugs are the most rapidly progressing drug type under the target SGLT2, with a dominant presence in the approved stage.
The United States, European Union, Japan, and China are the countries/locations developing fastest under the current target. China, in particular, has shown progress in the development of drugs for the target SGLT2.Overall, the target SGLT2 presents a competitive landscape with a focus on addressing Diabetes Mellitus, Type 2. The future development of this target is expected to continue with advancements in R&D efforts and increased competition among pharmaceutical companies.
Key Drug: Dapagliflozin
Dapagliflozin is a small molecule drug that belongs to the class of SGLT2 inhibitors. It is primarily used in the treatment of various diseases related to the cardiovascular system, urogenital system, immune system, endocrinology, and metabolic disorders. The drug has been approved for multiple indications, including diabetes mellitus type 1 and type 2, chronic kidney diseases, heart failure, myocardial infarction, kidney diseases, and cardiovascular diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
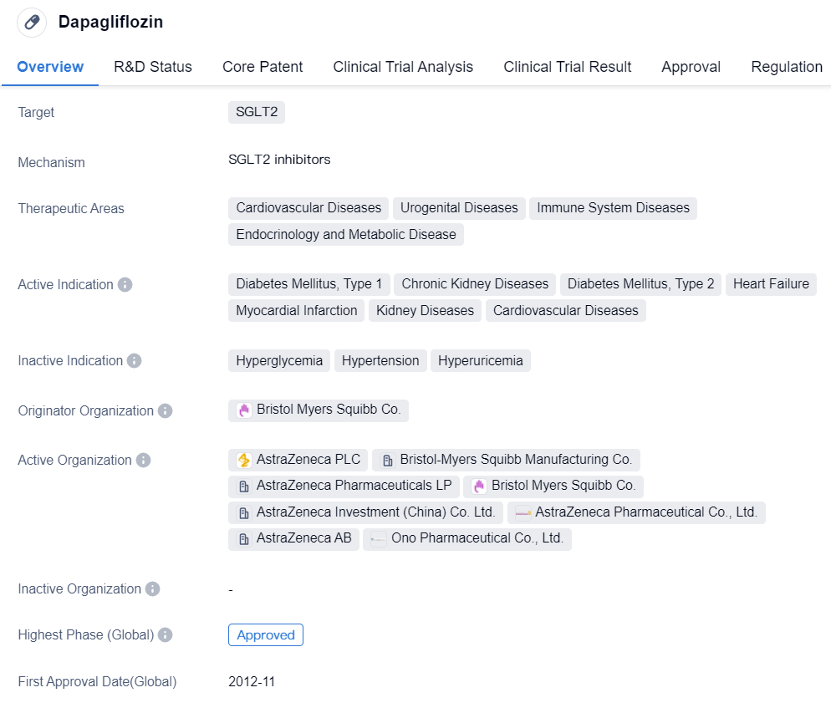
Dapagliflozin was first approved in the European Union in November 2012, making it available for patients in that region. The drug is developed by Bristol Myers Squibb Co., a renowned pharmaceutical organization. It has also received approvals in other countries, including China, indicating its global acceptance and recognition.
In summary, dapagliflozin targets the SGLT2 protein and has been approved for multiple indications, including diabetes mellitus, chronic kidney diseases, heart failure, and cardiovascular diseases. Its regulatory designations and global approvals reflect its potential in addressing unmet medical needs and providing advancements in the field of biomedicine.
Canagliflozin
Canagliflozin is a small molecule drug that belongs to the class of sodium-glucose cotransporter 2 (SGLT2) inhibitors. The active indications for Canagliflozin include diabetic nephropathies, cardiovascular diseases, type 2 diabetes mellitus, heart failure, type 1 diabetes mellitus, and obesity. These conditions are prevalent worldwide and pose significant health challenges to patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
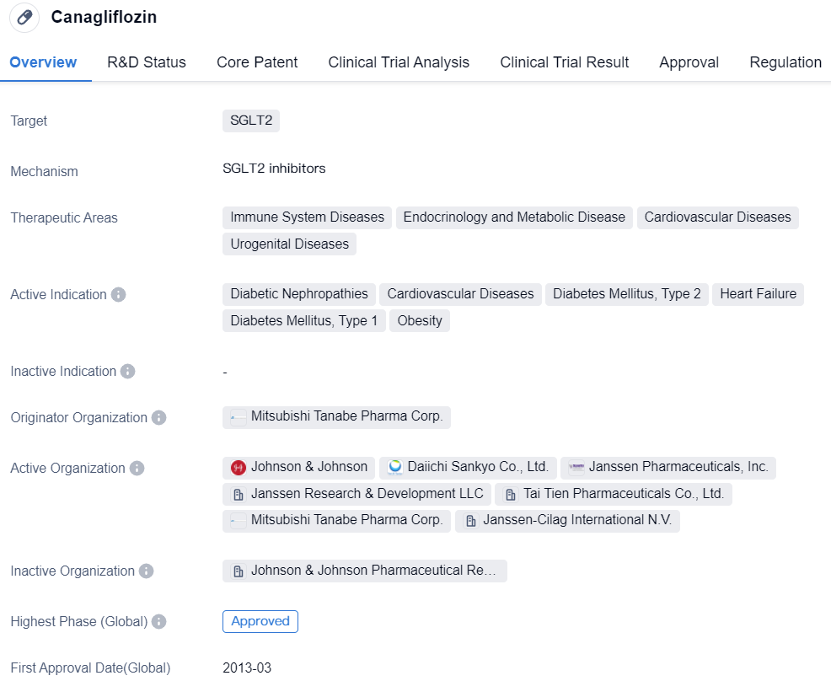
The drug was developed by Mitsubishi Tanabe Pharma Corp., a renowned pharmaceutical organization. Canagliflozin has successfully completed the highest phase of clinical trials and has received approval both globally and in China. The first approval for Canagliflozin was granted in the United States in March 2013, indicating its early entry into the market.
In summary, Canagliflozin is used in the treatment of immune system diseases, endocrinology and metabolic diseases, cardiovascular diseases, and urogenital diseases. It has been approved globally and in China, with its first approval granted in the United States in 2013. Developed by Mitsubishi Tanabe Pharma Corp., Canagliflozin underwent a priority review, indicating its potential benefits and urgency in addressing various medical conditions.




