Sirius Therapeutics announces its request to start initial clinical trials for Factor XI siRNA
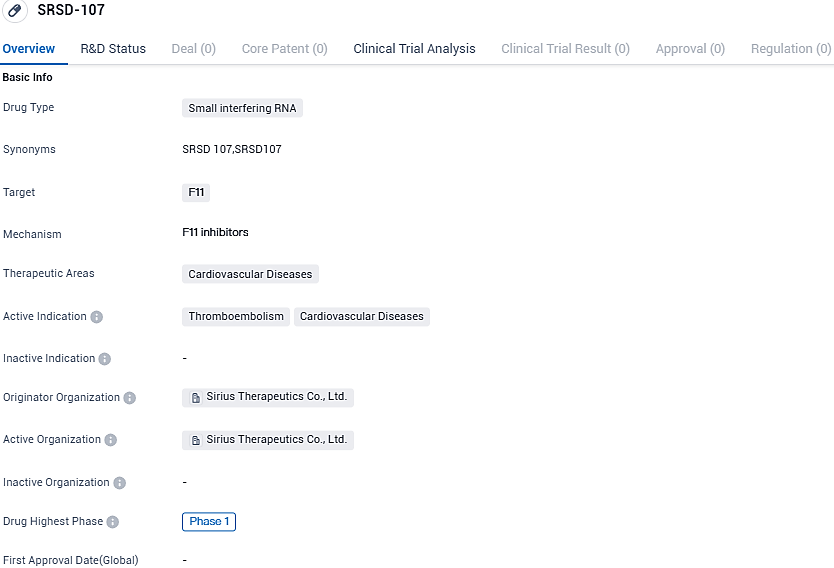
Sirius Therapeutics has filed a request in Australia to initiate a pioneering human clinical examination of SRSD107, its advanced siRNA therapeutic for inhibiting and addressing thromboembolic disorders. Primarily designed to target coagulation factor XI (FXI), SRSD107 is the first compound developed by Sirius to achieve a clinical phase of evolution on an international scale.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Since the inception of Sirius two years ago, we have made significant strides in the development of SRSD107 and other potential candidates in our portfolio. This submission signifies our shift from the discovery phase to clinical development," stated Sirius Therapeutics CEO, Dr. Qunsheng Ji. Dr.
Ji further commented, "The extraordinary durability of SRSD107's pharmacological effectiveness and safety profile indicate that it has the potential to be a superior and pioneering FXI siRNA therapeutic.
Our goal is to establish SRSD107 as a revolutionary medicine with a dosage routine of semiannually or possibly annually for patients requiring long-term anticoagulants." Thrombosis is the principal cause behind most myocardial infarction, ischemic stroke, and venous thromboembolism cases.
A study in The Lancet, discussing regional and global mortality rates, estimated that thromboembolic disorders caused one in every four fatalities globally. SRSD107 is a unique double-stranded small interfering ribonucleic acid, engineered by Sirius Therapeutics.
It precisely targets coagulation factor XI (FXI) mRNA and suppresses the expression of the FXI protein, thus obstructing the inherent coagulation pathway and encouraging anticoagulant and anti-thrombotic effects. Preclinical in vivo research revealed nearly a complete reduction of FXI concentrations for up to six months, with no reports of bleeding incidents after a single subcutaneous dose.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
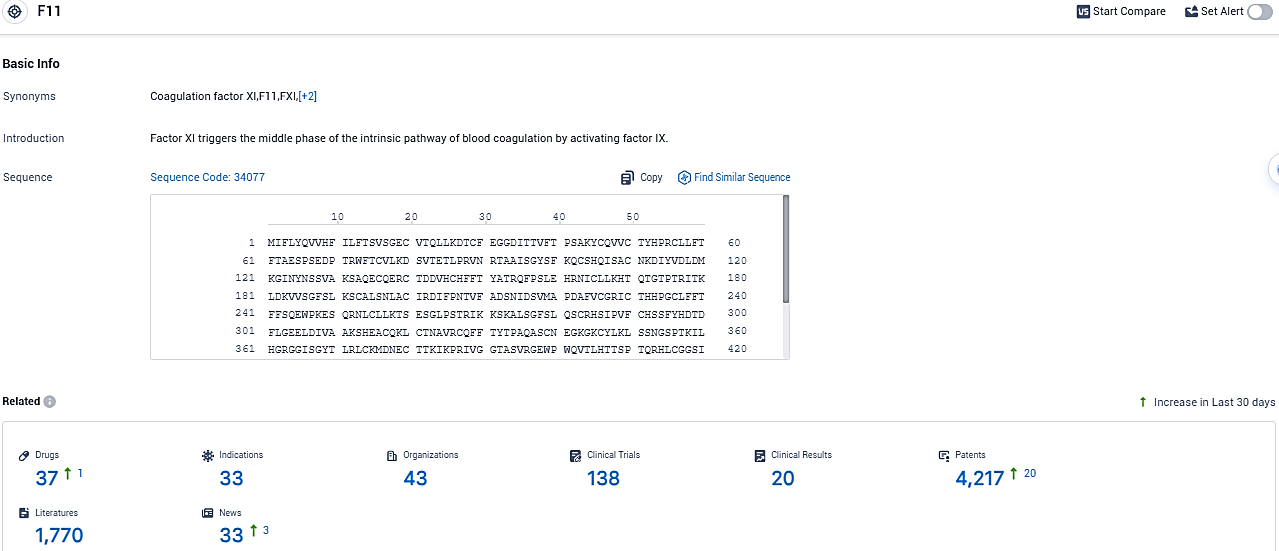
According to the data provided by the Synapse Database, As of November 18, 2023, there are 37 investigational drugs for the F11 target, including 33 indications, 43 R&D institutions involved, with related clinical trials reaching 138, and as many as 4217 patents.
SRSD-107 targets the F11 gene and is being investigated for its potential use in the treatment of cardiovascular diseases, particularly thromboembolism. The drug has reached Phase 1 of clinical development, indicating that it has undergone initial testing in humans. However, further research and clinical trials are needed to assess its effectiveness and safety.