Sosei Heptares to regain full rights to GSK4381406, a clinic-ready, novel oral GPR35 agonist for inflammatory bowel disease
Sosei Group Corporation has declared that talks to reclaim complete control of GSK4381406 have begun with GSK. This first-in-class, oral GPR35 agonist, characterized by its distinct selectivity, which is being cultivated under a Global Collaboration and License Agreement with GSK, could potentially serve as a novel therapeutic approach for Inflammatory Bowel Diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
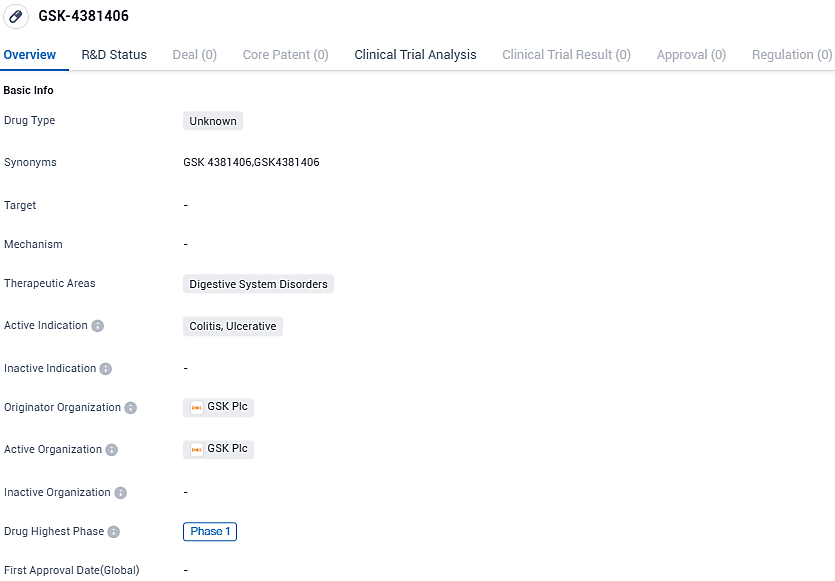
GPR35 is a crucial orphan G protein-coupled receptor that has a proven genetic link to IBD, a disease that lacks sufficient treatments for the millions of patients affected globally. Sosei Heptares developed GSK4381406 using its proprietary structure-based drug design platform and licensed it to GSK in 2020.
Ever since, both organizations have cooperatively advanced GSK4381406 under a collaborative development program, generating promising data from a mechanistic, preclinical and safety perspective. This data indicates the drug may enhance barrier function and alleviate visceral pain related to gastrointestinal disorders, such as ulcerative colitis and irritable bowel syndrome.
This valuable data led to Sosei Heptares and GSK obtaining clearance from the UK Medicines and Healthcare products Regulatory Agency in mid-2023 to introduce GSK4381406 into first-in-human studies.
Sosei Heptares’ approach is to further develop unique or best-in-class products during initial clinical stages and then find a collaborator for development and market launch in bigger markets. Following the company’s acquisition of Idorsia’s pharmaceutical operations in Japan and Korea, Sosei Heptares now possesses complete clinical development and market authorization capabilities in the Japan/Asia-Pacific regions.
We have maintained a fruitful alliance with GSK in the past years and we understand and respect its decision due to a shift in its immunology pipeline priorities. For Sosei Heptares, this represents a substantial opportunity which we have leveraged multiple times in recent years to regain and re-partner promising programs that have profited from external cooperation and investment to confirm their potential and advance them through development.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
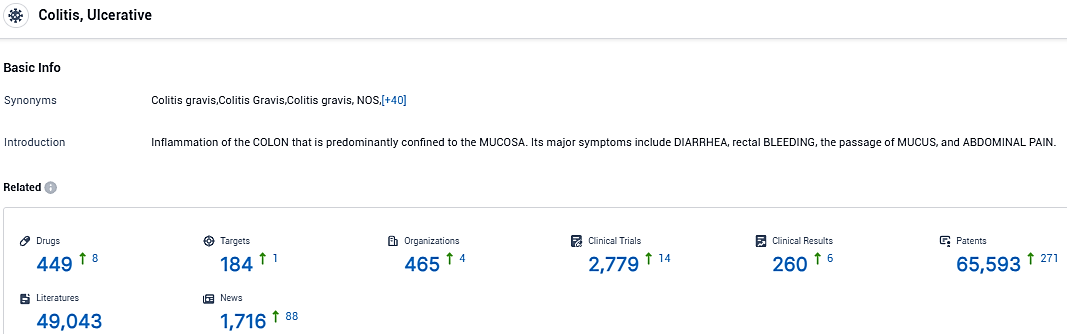
According to the data provided by the Synapse Database, As of November 30, 2023, there are 449 investigational drugs for ulcerative colitis, including 184 targets, 465 R&D institutions involved, with related clinical trials reaching 2779, and as many as 65593 patents.
GSK-4381406 is an investigational drug for the treatment of digestive system disorders, specifically colitis and ulcerative indications. It is currently in Phase 1 of clinical development, and further research is needed to determine its potential as a therapeutic option for patients suffering from these conditions.