Cabaletta Bio Announces Positive Early Results for CABA-201 Phase 1/2 Studies in Myositis and SLE
Cabaletta Bio, Inc., a biotechnology company in the clinical stage aiming to create and market the initial curative targeted cell therapies tailored for autoimmune disease patients, announced today encouraging early clinical data from the first two patients treated with CABA-201 in the Phase 1/2 RESET-Myositis and RESET-SLE trials.
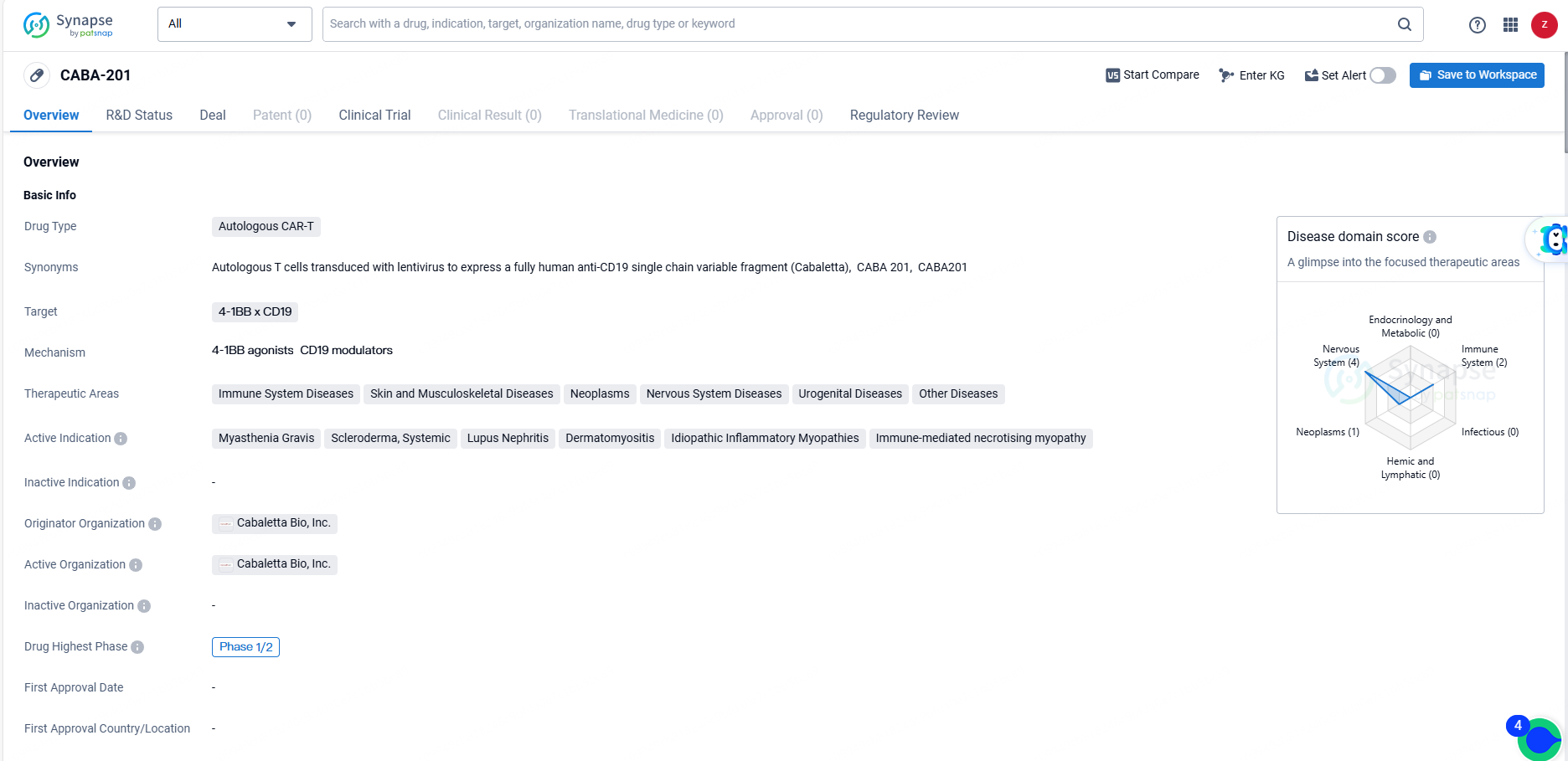
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
David J. Chang, M.D., Chief Medical Officer of Cabaletta, expressed optimism about the early safety, clinical, and translational data emerging from the RESET-Myositis and RESET-SLE studies. He highlighted that these findings provide crucial preliminary validation for the chosen clinical dose of CABA-201, suggesting it might effectively reset the immune system in autoimmune disease patients.
Dr. Chang further noted that with the recent initiation of enrollment for the RESET-SSc™ and RESET-MG™ studies, the inclusion of a juvenile myositis cohort in the RESET-Myositis trial, and the positive momentum from the initial clinical results, there is an eagerness to speed up enrollment in the RESET clinical program.
CABA-201 is a 4-1BB-containing fully human CD19-CAR T cell investigational therapy developed by Cabaletta. The therapy is designed to significantly but temporarily deplete CD19-positive B cells with a single infusion, potentially leading to an immune system reset and long-term remission without ongoing treatment in autoimmune disease patients.
Cabaletta is conducting four Phase 1/2 RESET trials to evaluate CABA-201, comprising a total of ten cohorts with six patients each. Each cohort receives a consistent, weight-based dose of 1 x 10^6 cells/kg after a preconditioning regimen of fludarabine and cyclophosphamide, in line with the dosage used in prior academic studies, and there is no requirement for dose escalation.
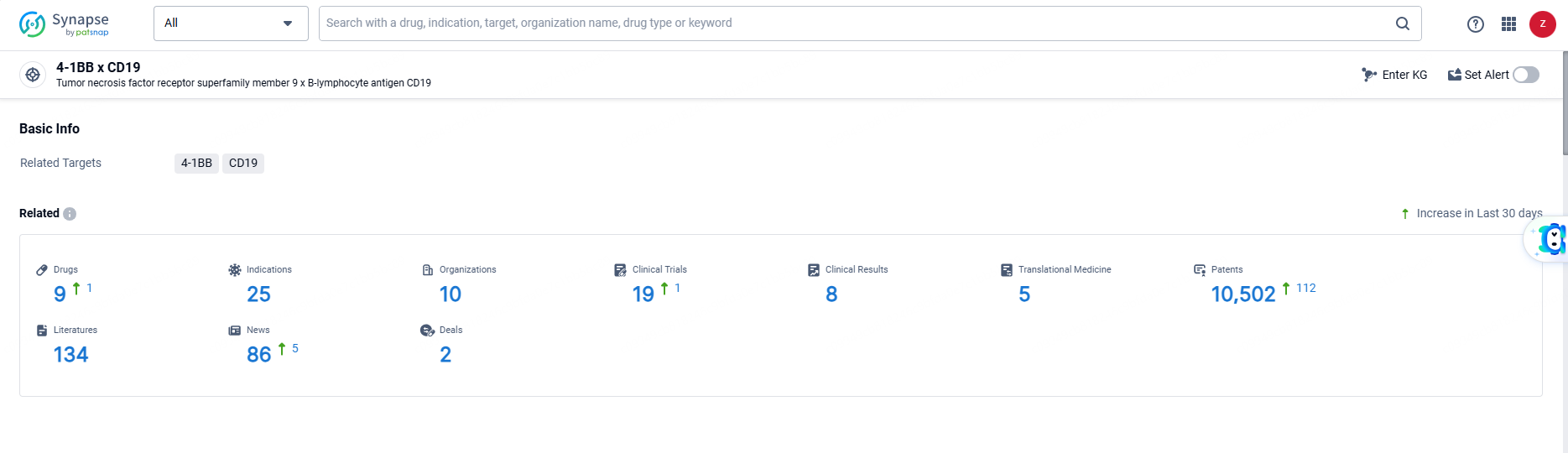
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 19, 2024, there are 9 investigational drugs for the 4-1BB and CD19 target, including 25 indications, 10 R&D institutions involved, with related clinical trials reaching 19, and as many as 10502 patents.
CABA-201 targets 4-1BB x CD19 and is being developed for the treatment of a wide range of therapeutic areas, including immune system diseases, skin and musculoskeletal diseases, neoplasms, nervous system diseases, urogenital diseases, and other diseases. CABA-201 has shown promise in its early-stage development, and its regulatory designations reflect the potential for expedited development and review. As the drug progresses through clinical trials, further data will be needed to assess its safety and efficacy in treating the targeted diseases.