Targeting the Amylin Receptor for Obesity Weight Reduction
Islet Amyloid Polypeptide (IAPP), also known as amylin, is a peptide hormone composed of 37 amino acids, secreted by the pancreatic beta-cells.
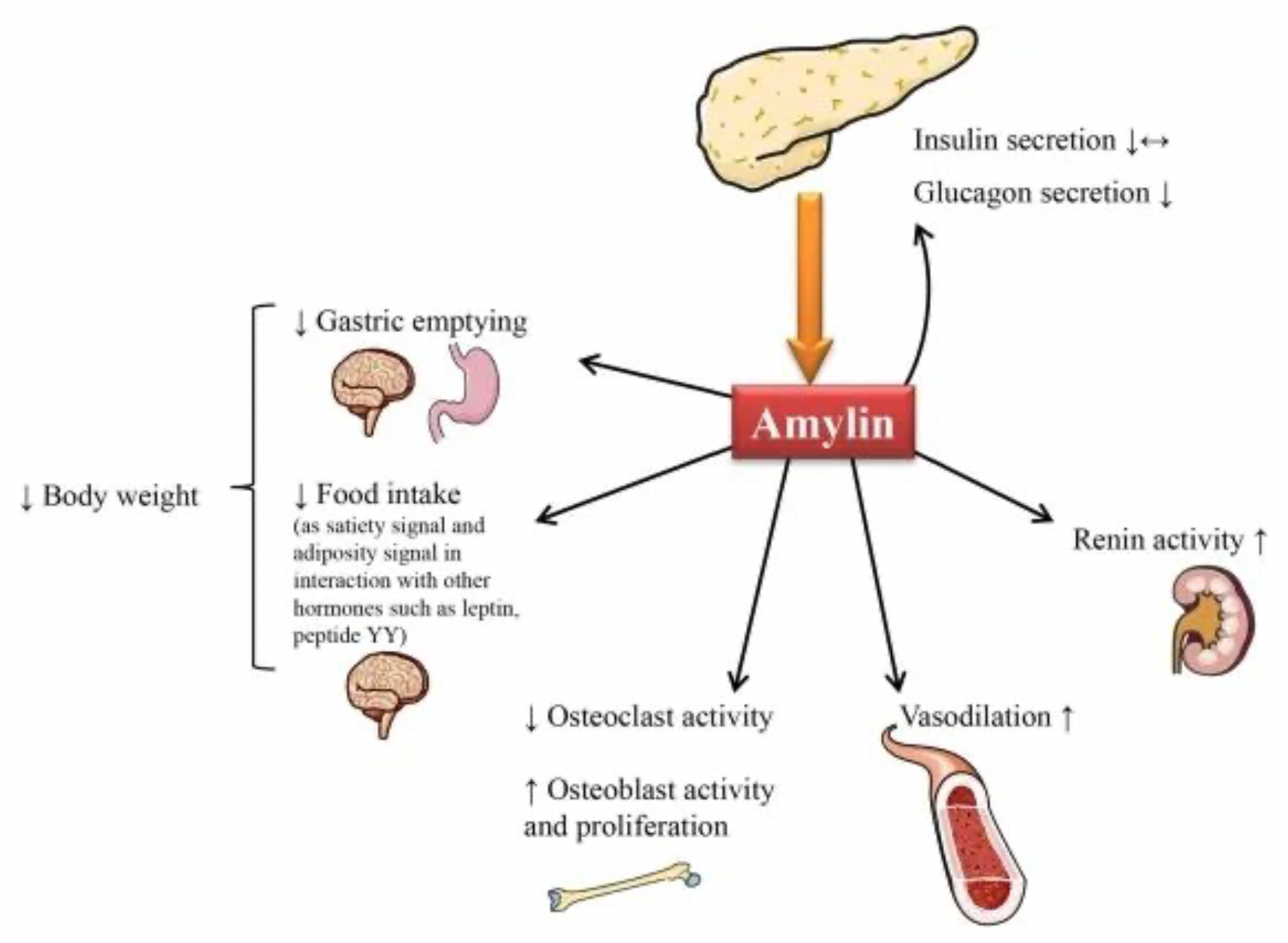
Amylin acts as an appetite suppressant. It inhibits gastrointestinal motility and delays gastric emptying after meals, thereby reducing appetite. The metabolic regulatory functions of amylin are mediated by the Amylin receptors (AMYRs), making these receptors an important pharmaceutical target in the treatment of diabetes and obesity.
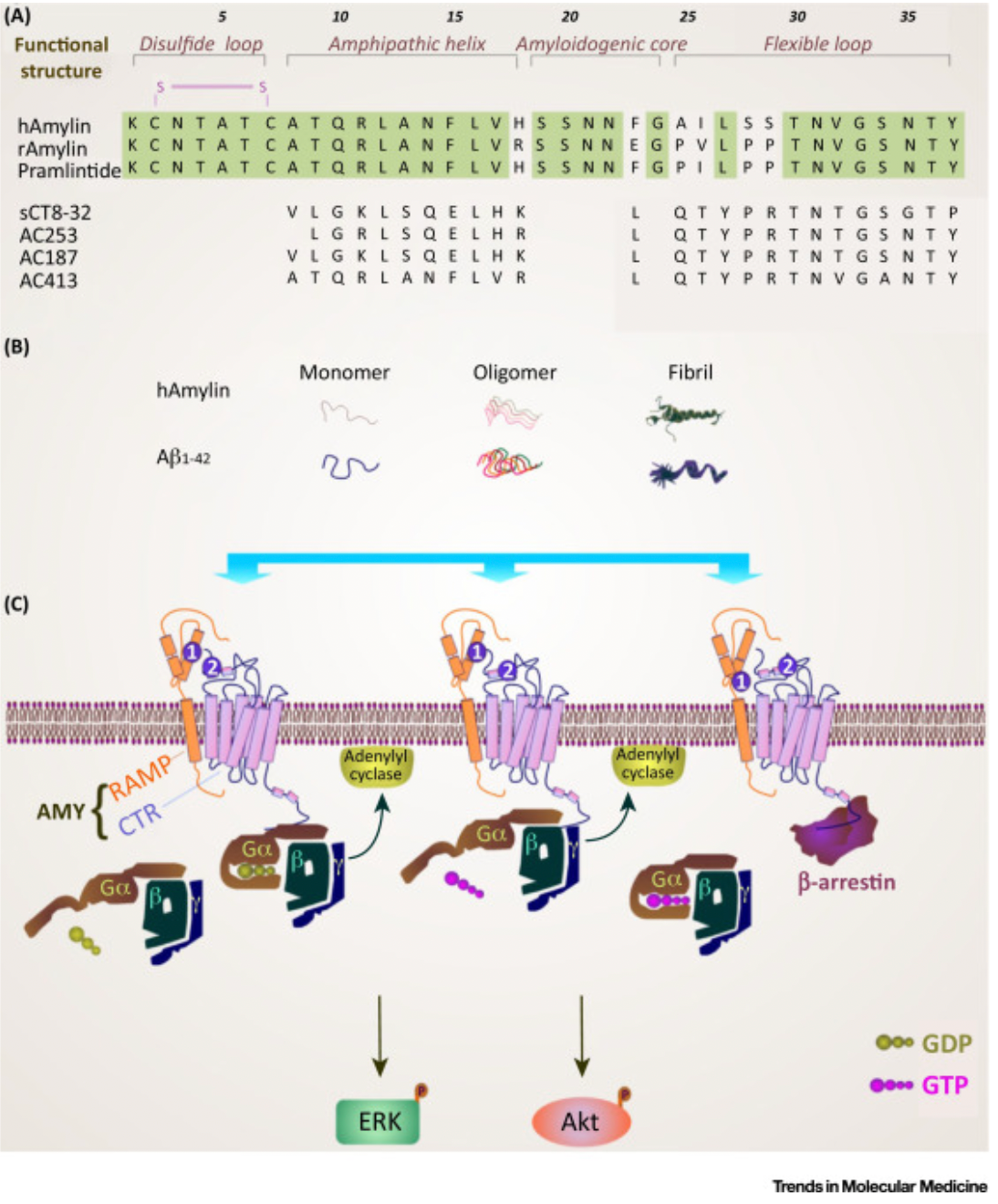
Taking Pramlintide as an example, it is a synthetic analog of human insulin composed of 31 amino acids. Developed by AstraZeneca, it was approved for market in 2005 for the treatment of type 1 diabetes. Due to the highly amyloidogenic nature of endogenous human insulin, researchers took inspiration from the proline residues in rat insulin, which are highly homologous but not amyloidogenic (known to disrupt the secondary structure), and incorporated them into the human insulin sequence to develop Pramlintide.
Insulin receptors are a class of special protein heterodimers, composed of a calcitonin receptor (CTR) combined with three homologous receptor activity-modifying proteins (RAMP 1,2,3), forming three subtypes (AMY1, AMY2, AMY3).
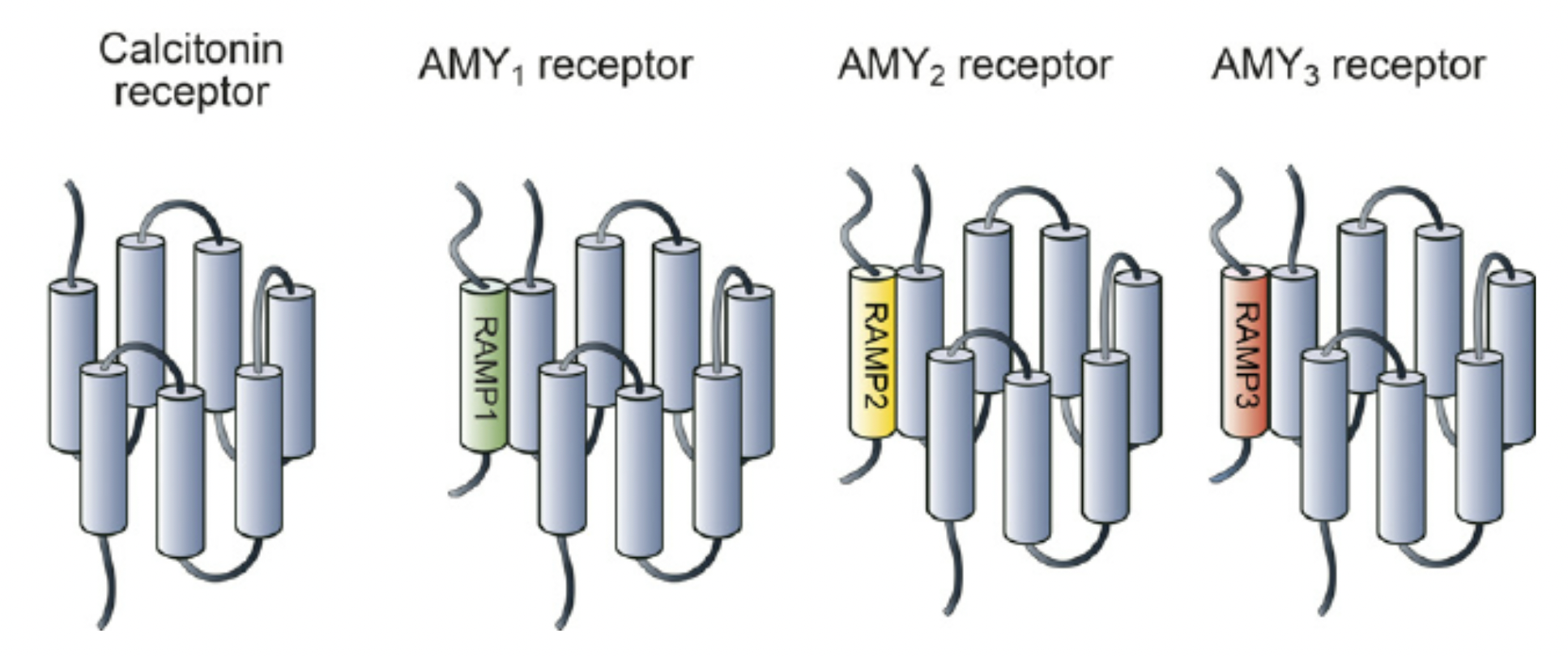
The cagrilintide developed by Novo Nordisk is a long-acting insulin analog. Studies have shown that cagrilintide can act on both the calcitonin receptor and three different subtypes of insulin receptors. Additionally, clinical results indicate that cagrilintide possesses effective weight-reducing properties.